Cardiogenic shock (CS) is a life-threatening condition characterized by cardiac pump dysfunction resulting in end-organ hypoperfusion and hypoxia.1,2 Although multiple treatment strategies have been explored in clinical trials, optimal therapy for CS is still debatable and mortality remains substantially high at around 50%.1 This need for improved treatment, together with a growing burden of patients with congestive heart failure who are refractory to guideline-directed medical therapy, has led to the emergence of a variety of mechanical circulatory support (MCS) devices that can be used to restore systemic perfusion and break the spiral of myocardial ischaemia, allowing cardiac recovery in the short term and/or as a bridge to transplantation or as destination therapy in refractory heart failure.3 MCS may also be used during high-risk procedures such as complex percutaneous coronary interventions (PCI) or thrombolysis for pulmonary embolism. Several different classes of MCS devices have been evaluated in clinical practice and are distinguished by haemodynamic characteristics, site of blood draw and return, technique of insertion and the utilization of gas exchange units. As a group, these MCS devices improve cardiac output and blood pressure; however, their specific mechanical characteristics produce varying haemodynamic effects. It is estimated that at least 2,000 MCS device implantations are performed annually in the USA.4 While there has been an exponential increase in MCS use in the USA over the past few years, data from European counterparts revealed a declining trend.4–6 The goal of this review article is to provide a brief overview of the types of currently available MCS devices and the indications for their use.
Rationale for use of mechanical circulatory support
CS is characterized by low cardiac index (<1.8 L/min/m2 without support and <2.0-2.2 L/min/m2 with support), systolic blood pressure <90 mmHg and signs of systemic hypoperfusion such as elevated lactic acid in the absence of hypovolemia (Figure 1).7 Despite remarkable advances in the field of cardiovascular disease over the past decade, only marginal improvements have been noted in CS outcomes, including mortality, and there is a wide variation in clinical outcomes based on aetiology. The mortality rate for cardiogenic shock complicating acute myocardial infarction (AMI-CS) ranges from 50% to 60%, while acute decompensated heart failure-related CS mortality is around 40%.8 In addition, the trajectory of heart failure progression is wide with varying phenotypic presentations, ranging from minimal or no symptoms to refractory end-stage heart failure. Thus, it remains difficult to prove the therapeutic benefit of conventional treatments in this heterogeneous patient population. These unresolved pitfalls prompted the development of a robust and dynamic classification system by the Society of Cardiovascular Angiography and Interventions that helps to quickly triage patients with CS based on symptom severity and comorbidity burden (Table 1).7 Patients with severe CS refractory to conventional pharmacological treatments should be carefully selected for trial of MCS devices. The three most important goals of MCS devices are: 1) to increase systemic perfusion, 2) to enhance coronary perfusion and 3) to reduce left ventricle (LV) filling pressures, LV wall stress and myocardial oxygen consumption. Clinical scenarios where MCS devices have been used to provide haemodynamic support or used as a bridge to recovery or as destination therapy are included in Table 2.



The timing of MCS device insertion may be critical in certain clinical situations.9,10 In AMI-CS, MCS devices should be inserted as soon as possible if the initial resuscitative attempts with conventional pharmacological support fail, and preferably before PCI.11 This was explored in a multidisciplinary team-based protocol, the National Cardiogenic Shock Initiative, which used a standardized treatment algorithm to improve the effectiveness of MCS devices in AMI-CS.12 The initial results showed that the use of a shock protocol emphasizing rapid initiation of MCS prior to PCI in AMI-CS was associated with improved survival to hospital discharge (74%).13 The National Cardiogenic Shock Initiative also supports the utilization of invasive haemodynamic monitoring to tailor the treatment decisions post-procedure. The American Heart Association scientific statement on CS supports the use of right heart catheterization to obtain important objective haemodynamic data.1 More recently, a retrospective analysis by the Cardiogenic Shock Working Group showed that patients who had complete haemodynamic data available by right heart catheterization had lowest in-hospital mortality compared with those who did not have such haemodynamic data prior to initiation of MCS.14,15 As such, measurement of haemodynamic data through right heart catheterization can assist in tailoring the management of CS, including escalation to MCS devices in appropriate clinical scenarios.16
Haemodynamic monitoring using pulmonary artery catheterization
Direct parameters
Although right heart catheterization by itself was not associated with any mortality benefit, the haemodynamic parameters obtained from such a procedure, such as central venous pressure (CVP), pulmonary artery systolic pressure (PASP), pulmonary artery diastolic pressure (PADP), pulmonary artery occlusion pressure or pulmonary capillary wedge pressure (PCWP) and cardiac output/cardiac index, have been correlated with mortality outcomes.17 These parameters can be used to calculate pulmonary vascular resistance and quantify right ventricle (RV) function.18
Derived parameters
Cardiac power output (CPO) and cardiac power index (CPI) are two important derived parameters that have been strongly correlated to the risk of in-hospital mortality in CS.19 CPO and CPI quantify the external mechanical work done by the LV. CPO ($\frac{MAP–RAP × CO}{451} $)<0.6 watts is predictive of worse outcomes in AMI-CS, despite guideline-directed treatment.19,20
In the same way as the above indices serve as surrogates for LV function, indices such as RV stroke work, RV stroke work index and CVP/PCWP ratio quantify the RV function. A CVP/PCWP ratio >0.86 signifies impaired RV function, provided CVP is elevated above normal.21 Similarly to LV parameters, RV CPO and RV CPI can be measured, although they are less commonly used in clinical practice.22 Another important measure that has gained significant attention in recent times is the pulmonary artery pulsatility index ($\frac{PASP–PADP}{CVP} $), which has been found to be more specific for evaluation of the degree of RV dysfunction, with values <0.9 indicating significant RV dysfunction and need for RV mechanical support.21,23
Classification of cardiogenic shock
CS can be classified based on the ventricle affected: 1) LV dominant, 2) RV dominant or 3) biventricular failure (Figure 2). LV-dominant CS is characterized by high PCWP (>18 mmHg) with normal or reduced CVP (<14 mmHg) secondary to reduced LV contractile function. RV-dominant CS is characterized by elevated CVP (>14 mmHg) with normal or reduced pulmonary artery pressure and PCWP (<18 mmHg) in the setting of preserved LV contractility. Biventricular shock is characterized by elevated CVP (>14 mmHg), normal or elevated PCWP (>18 mmHg) and hypotension, along with reduced LV function. At least 40% of patients diagnosed with LV-dominant CS, in fact have biventricular failure.24

Types of mechanical circulatory support
A variety of US Food and Drug Administration (FDA)-approved devices and investigational pumps are currently available for temporary or long-term haemodynamic support in the USA. The majority of the available pumps were designed for LV support. Durable MCS for RV support are not currently approved by the FDA.25 Depending on the duration of mechanical support, these devices can be classified as short term/temporary or long term/durable.26 Depending on their mechanism of action, pumps can be classified as volume displacement pumps, centrifugal or axial flow pumps.
Short-term mechanical circulatory support devices
Short-term MCS devices can be further classified according to the type of ventricular assistance: LV support devices, RV support devices or biventricular support devices (Table 3, Figure 3).


Left ventricle support devices
Intra-aortic balloon pump
The intra-aortic balloon pump (IABP) has been the most commonly used MCS device in cardiac catheterization laboratories since its introduction in 1960.27 It uses counter pulsations of a balloon that sits in the descending aorta and decreases impedance to LV systolic ejection, thereby improving coronary perfusion and cardiac output (CO). Although earlier studies suggested a modest increase in CO of 0.5 L/min, more recent data suggest that there is no improvement in either CO or haemodynamic parameters with IABP.28–30 In addition, because a drop in mean arterial pressure (MAP) may occur, use of vasopressor agents may be needed. As such, IABP is used to augment coronary and systemic perfusion, rather than as an effective MCS device. The ease of insertion, along with widespread familiarity, led to its rapid dissemination prior to the availability of other MCS devices. However, several studies reported a progressive decline in the use of IABP in recent years.31–33 The device includes two main components: a double-lumen 7.5 Fr to 8.0 Fr catheter and a pump console to control the balloon. The inner lumen accommodates the guidewire and transduces aortic pressure for monitoring. The majority of complications associated with IABP are vascular complications such as stroke and limb ischaemia. Other complications include thrombocytopaenia due to platelet deposition on the IABP surface and infection. Contraindications for IABP placement include severe aortic regurgitation, aortic aneurysm, aortic dissection and peripheral vascular disease.
The IABP-SHOCK II trial (Intra-aortic balloon counterpulsation in patients with AMI-CS; ClinicalTrials.gov identifier: NCT00491036), which compared IABP with medical therapy in patients with AMI-CS, showed that IABP was not associated with any short- or long-term mortality benefit.34,35 The trial investigators attributed this to the non-salvageable effect of IABP on myocardial recovery. IABP was compared head-to-head against percutaneous MCS devices such as the TandemHeart, Impella 2.5 and Impella CP in clinical situations such as AMI-CS and high-risk PCI.36–40 There was no short-term all-cause mortality difference between the two groups in these randomized trials. A meta-analysis of trials comparing IABP versus Impella or TandemHeart in CS showed similar results for 30-day mortality, although percutaneous MCS devices were associated with an increase in MAP compared with IABP.41 Contemporary European guidelines recommend against the use of IABP in AMI-CS.16,42,43
Left ventricle to arterial circulatory support
Impella
The Impella® devices (Abiomed, Danvers, MA, USA) are a series of non-pulsatile micro axial flow pumps that can provide haemodynamic support up to 5 L/min, and about 50,000 devices have been inserted since FDA 510(k) clearance in 2008. There are three classes based on the level of LV support: 1) Impella 2.5® (2.5 L/min, 12 Fr system), 2) Impella cardiac power (CP)® (3.5 L/min, 14 Fr system), 3) Impella 5.5® (5.0 L/min, 21 Fr system). The Impella 2.5 and CP can be placed percutaneously, whereas the Impella 5.5 requires surgical cut-down and the device is delivered through a Dacron side graft on the axillary or femoral artery. All three devices are FDA approved for haemodynamic support in CS, and the Impella 2.5 and Impella CP are also approved for use during high-risk PCI. The Impella expandable cardiac power (ECP)® device, which can be implanted through a 9 Fr sheath and can provide flows up to 3.5 L/min, is currently being investigated in a clinical trial.44 The device configuration includes: 1) catheter, 2) purge system and 3) automated controller. It works on the Archimedes principle of shunting blood from the LV into the aorta across the aortic valve and results in three primary haemodynamic effects: 1) unloads LV through reduction of left ventricular end diastolic pressure and left ventricular end diastolic volume, 2) reduces PCWP and RV afterload and 3) increases MAP, CO and CPO (Figure 4). The Impella technology is load dependent, where pump flow decreases with increasing ventriculo-aortic pressure gradient. Anticoagulation with heparin is required during Impella use. Severe peripheral vascular disease and very severe aortic stenosis may preclude its implantation. The device is contraindicated in the presence of LV thrombus and mechanical aortic valves. Complications include bleeding, vascular injury, infection, haemolysis and pump migration.

Much of the evidence relating to Impella use comes from observational studies. One of the largest studies to date retrospectively reviewed 154 patients with AMI-CS from the USpella Registry.11 The results showed that early initiation of Impella 2.5 prior to PCI improved survival rates and also complete revascularization compared with those who received it post-PCI. The IMPRESS trial (Impella versus IABP reduces mortality in STEMI patients treated with primary PCI in severe cardiogenic shock) randomized 24 patients with AMI-CS to either Impella CP or IABP, and found no difference in 30-day and 6-month mortality between the two groups.39 In the ISAR-SHOCK trial (Efficacy study of LV assist device to treat patients with cardiogenic shock; ClinicalTrials.gov identifier: NCT00417378), 25 patients with AMI-CS were randomized to receive either Impella 2.5 (n=12) or IABP (n=13).38 Impella 2.5 was associated with greater increase in cardiac index (CI) (Impella 2.5: DCI=0.49 ± 0.46 L/min/m2; IABP: DCI=0.11 ± 0.31 L/min/m2; p=0.02), although there was no difference in 30-day mortality between the two groups.38 However, the small sample sizes of these earlier studies precludes any definitive conclusions regarding the use of either of these devices in AMI-CS. An ongoing randomized controlled trial (RCT), DTU-STEMI (Primary unloading delayed reperfusion in ST-elevation myocardial infarction: The STEMI-DTU trial; ClinicalTrials.gov identifier: NCT03947619), plans to enrol 668 patients with anterior STEMI and randomize them to receive the Impella CP prior to PCI or to undergo standard institutional treatment.45 In addition to AMI-CS, Impella devices have also been used to provide MCS in patients receiving high-risk PCI. The PROTECT II trial (Protect II, a prospective, multicenter randomized controlled trial; ClinicalTrials.gov identifier: NCT00562016), one of the largest RCTs in patients with high-risk PCI, randomized 452 patients to receive the Impella 2.5 (n=226) or IABP (n=226) during high-risk PCI.46 Although the trial was not completed due to futility, the primary endpoint of a 30-day composite outcome of 11 adverse events was not significantly different between the two groups. The single-arm PROTECT III study (Protected PCI study; ClinicalTrials.gov identifier: NCT02831881), conducted as a part of post-marketing surveillance of the Impella 2.5/Impella CP for high-risk PCI showed that there was a lower composite endpoint of major adverse cardiac and cerebrovascular events with both the Impella 2.5 and Impella CP, compared with such events observed among the PROTECT II trial participants who received the Impella 2.5.47 The ongoing PROTECT IV (Impella®-supported PCI in high-risk patients with complex coronary artery disease and reduced left ventricular function; ClinicalTrials.gov identifier: NCT04763200) RCT is currently enrolling patients who will be randomized to undergo high-risk PCI with or without the Impella 2.5.48
HeartMate percutaneous heart pump
HeartMate® percutaneous heart pump (PHP) (Abbott Laboratories, Abbott Park, IL, USA [formerly Thoratec Corp., Pleasanton, CA, USA]) is a second-generation, catheter-based microaxial three-blade Impeller pump that can provide flows up to 4 to 5 L/min.49 It has received a Conformité Européenne (CE) mark for short-term use in the Europe. It is delivered percutaneously into the femoral artery via an integrated 14 Fr sheath. The pump expands to 24 Fr once it is placed across the aortic valve and pumps the blood from LV to the aorta. The HeartMate pump was evaluated in the SHIELD I trial, which enrolled 50 patients who were at risk of haemodynamic compromise due to LV dysfunction during high-risk PCI.50 None of the patients met the primary performance endpoints, which included haemodynamic compromise during PCI and a composite of major adverse events.50 Following this trial, the SHIELD II trial (SHIELD II clinical investigation; ClinicalTrials.gov identifier: NCT02468778) was initiated to compare the HeartMate pump with the Impella 2.5, but the trial was terminated due to reports of device malfunction.51,52
Right atrium to systemic arterial circulatory support
Extracorporeal membrane oxygenation
Extracorporeal membrane oxygenation (ECMO) is an MCS method that can be used to provide cardiopulmonary support for prolonged periods. There are two varieties available: venovenous (VV) ECMO and venoarterial (VA) ECMO. VA-ECMO provides both respiratory and haemodynamic support in acute cardiorespiratory failure and has also been used to assist cardiopulmonary resuscitation in cardiac arrest, known as extracorporeal cardiopulmonary resuscitation.53–55 The device configuration includes three components: 1) membrane oxygenator and heat exchanger, 2) centrifugal or roller pump, and 3) drainage or perfusion cannula. Cannulation can be done centrally or peripherally. Central cannulation includes a drainage cannula in the right atrium and a perfusion cannula in the ascending aorta. However, this approach needs a sternotomy or thoracotomy; therefore, it is most often used in patients for cardiac surgery who are not able to be weaned off cardiopulmonary bypass. Peripheral cannulation includes a drainage cannula in the jugular or femoral vein, and a perfusion cannula in the femoral, axillary, subclavian or sometimes in the carotid artery. The blood is drawn from the venous system at the right atrium or inferior vena cava and returned to the arterial system through a central or peripheral cannulation site after gas exchange. As a result, there is an increase in afterload and stroke work without any significant reduction of LV wall stress (Figure 5). Therefore, venting is often required to decompress the LV and prevent dilation. Some strategies include atrial septostomy, surgical LV vent, IABP or percutaneous left ventricular assist device (LVAD).56 As such, anticoagulation is required and most often achieved through heparin. Other complications include upper body hypoxaemia, bleeding, infection and limb ischaemia. One of the interesting clinical complications is Harlequin syndrome, which is reported in 8.8% of cases.57 Harlequin syndrome is observed in patients with preserved LV ejection fraction when the mixing point of oxygenated blood from ECMO and deoxygenated blood from native LV is below the level of origin of the left carotid artery. This clinical syndrome is also referred to as differential hypoxia and north–south syndrome.58 Contraindications to its use include multiorgan failure, prolonged cardiopulmonary resuscitation, aortic dissection and severe aortic regurgitation.

Although no RCTs have evaluated ECMO, observational data from large registries such as the Extracorporeal Life Support Organization showed a survival-to-hospital-discharge rate of 40.2% among 756 patients who received ECMO for AMI-CS.59 In another study using data from the same registry, the survival-to-hospital-discharge rate was 29% among 2,885 adults who received ECMO for extracorporeal cardiopulmonary resuscitation.60 Lemor et al. compared the use of the Impella versus ECMO in patients with AMI-CS using the Nationwide Inpatient Sample database. Although they found an increased in-hospital mortality among patients who received ECMO, these patients were much sicker compared with those who received the Impella.61 In another study by Vallabhajosyula et al. using the same database, there was a steady increase in the use of ECMO for AMI-CS, with concomitant decrease in in-hospital mortality from 2010 to 2014.62 RCTs are necessary to further evaluate the role of ECMO in AMI-CS. The ongoing ANCHOR RCT (Assessment of ECMO in acute myocardial infarction cardiogenic shock; ClinicalTrials.gov identifier: NCT04184635), will compare VA-ECMO plus IABP with VA-ECMO alone in patients with AMI-CS.63 Another ongoing trial, ECLS-SHOCK (Extracorporeal life support in cardiogenic shock; ClinicalTrials.gov identifier: NCT03637205), has been designed to evaluate whether VA-ECMO in addition to revascularization is beneficial among patients with AMI-CS compared with no VA-ECMO support.64 The 2017 American Heart Association scientific statement on management of CS supports the use of VA-ECMO in clinical scenarios with poor oxygenation that is not expected to improve with an alternative MCS device.1
Left atrium to systemic arterial circulatory support
TandemHeart
TandemHeart® (CardiacAssist, Inc., Pittsburgh, PA, USA) is a centrifugal continuous flow pump that can provide flows ranging from 3.5 L/min with a 15 Fr cannula to 5 L/min with a 19 Fr cannula. This is also the first totally percutaneous biventricular MCS device to come to market. It is FDA approved for 6 hours of use and CE marked for use up to 30 days. As the name suggests, the device works in tandem or parallel with the LV using a continuous centrifugal pump that circulates oxygenated blood from the left atrium to the lower abdominal aorta or iliac arteries. The device system includes: 1) inflow cannula (21 Fr) placed transeptally into the left atrium, 2) outflow cannula (15 Fr or 17 Fr) placed into the femoral artery, 3) centrifugal pump, and 4) control console. As the blood is directly withdrawn from the left atrium, the device unloads the LV by reducing left ventricular end diastolic pressure, left ventricular end diastolic volume, stroke work and myocardial oxygen demand (Figure 6). Common complications associated with this device include bleeding, thromboembolism and limb ischaemia. Additionally, complications associated with transseptal puncture, including cardiac wall perforation, aortic root puncture, pericardial effusion or tamponade, can occur. Contraindications include aortic regurgitation and peripheral vascular disease. Unlike the Impella, the TandemHeart can be used in the presence of LV thrombus, as there is no cannula in the LV.

Thiele et al. first reported the use of the TandemHeart in 18 patients with AMI-CS.65 In a small multicentre RCT including 33 patients, Burkhoff et al. compared the TandemHeart with IABP placed within 24 hours of developing CS after AMI (70%) or decompensated heart failure (30%).37 Compared with IABP, the TandemHeart was associated with a greater increase in cardiac index and MAP, and a greater decrease in PCWP; however, there was no difference in 30-day mortality and adverse events.37 In another study, Thiele et al., compared the TandemHeart with IABP in AMI-CS and observed greater improvement in haemodynamics with the TandemHeart compared with IABP, with no significant difference in 30-day mortality.36 Definitive conclusions cannot be drawn based on these trial results owing to their small sample sizes. The 2015 Society of Cardiovascular Angiography and Interventions/American College of Cardiology/American Heart Association/Heart Failure Society of American guidelines on the use of percutaneous MCS devices suggest the TandemHeart can be considered in the following clinical scenarios:
1) severe LV dysfunction (ejection fraction <35%), 2) CS unresponsive to the Impella 2.5 or Impella CP, 3) acute mechanical complications of myocardial infarction such as acute mitral regurgitation and ventricular septal rupture.66
The manufacturer of the TandemHeart introduced a similar device to support the RV, known as the TandemLife Protek Duo® (TPD; TandemLife, Pittsburgh, PA, USA). The inflow cannula is positioned in the right atrium and the outflow portion is in the pulmonary artery. In patients with coexisting respiratory failure, an oxygenator can be added to provide better oxygenation compared with VV-ECMO. This configuration has been used in the management of RV failure after LVAD implantation.
CentriMag and Rotaflow
The CentriMag® left ventricular assist system (Abbott Laboratories, Chicago, IL, USA) and the Rotaflow® (Maquet Cardiopulmonary AG, Hirrlingen, Germany) are magnetically levitated extracorporeal centrifugal flow pumps that can provide flows of up to 10 L/min.67 The Rotaflow pump is suspended on sapphire bearings, while the CentriMag pump does not have any bearing or shafts. Both these devices require surgical cut-down with the outflow (22 Fr) and inflow (32 Fr) cannulas positioned in the left atrium/aorta or right atrium/pulmonary artery, respectively. These pumps produce a continuous non-pulsatile blood flow with minimal contact between rotor and bloodstream. They are extremely preload and afterload sensitive. They are FDA approved for use up to 6 hours as LV assistance, while they can be used up to 30 days for RV assistance.68,69 Additionally, the CentriMag is also CE marked for use up to 30 days for any indication.70 An oxygenator can be spliced into the tubing system, allowing the addition of ECMO support to device configuration.
Much of the evidence related to their use comes from case reports and case series. Borisenko et al. performed a meta-analysis of 53 studies in which the CentriMag was used as LVAD (72%) or as part of ECMO (25%).71 The 30-day survival rate ranged from 41% to 66%. In another study by John et al., among 38 patients who received the CentriMag for AMI-CS (n=14), RV failure after LVAD implantation (n=12) or post-cardiac surgery (n=12), 30-day survival rate was 44% when the device was used for biventricular support and 58% when used for RV support.72 As such, the CentriMag has been commonly used in post-cardiotomy CS or as step-up therapy when haemodynamic support is inadequate with peripheral devices. In contrast, the current available evidence for the use of the Rotaflow comes from case reports/series.73–75
Right ventricle support devices
Acute RV failure can occur during CS or postoperatively after LVAD placement. MCS devices for RV failure can be inserted surgically or percutaneously.76,77 They can be divided based on flow into axial flow pump (Impella RP) or centrifugal flow pump (TandemHeart right ventricular assist device [TH-RVAD], Protek Duo, and VA-ECMO).
Impella RP
The Impella RP® (Abiomed , Danvers, MA, USA) is a minimally invasive, 22-Fr three-dimensional catheter-based microaxial flow pump that can be used for up to 14 days. The blood is aspirated from the inflow cannula placed in the inferior vena cava and ejected through the outflow cannula placed in the pulmonary artery. It can provide flow of up to 4 L/min and unloads the RV. The initial successful implants of the Impella RP were reported outside the USA in the setting of cardiac surgery. The efficacy and safety were evaluated in the RECOVER RIGHT (The use of Impella RP support system in patients with right heart failure; ClinicalTrials.gov identifier: NCT01777607) trial, in which the Impella RP was used for refractory RV failure after cardiac surgery in 18 patients and after LVAD implantation in 12 patients.78 The device immediately reduced CVP and improved cardiac index and had an overall 30-day survival rate of 73.3%. The Impella RP has also been used to provide haemodynamic support in patients with malignant ventricular arrhythmias and severe mitral regurgitation.79,80 Complications associated with use are similar to any other Impella device. Contraindications to use include tricuspid regurgitation and pulmonary regurgitation.
TandemHeart right ventricular assist device and Protek
Duo cannula
The TandemHeart right ventricular assist device (TH-RVAD®) (CardiacAssist, Pittsburgh, PA, USA) is an extracorporeal centrifugal flow pump that pumps the blood from the inflow cannula placed in the right atrium to the outflow cannula in the pulmonary artery. Both are venous cannulas, generally accessed through the left and right femoral veins, respectively. Alternatively, an internal jugular vein can be accessed to place the outflow cannula. TH-RVAD is not currently FDA approved for RV support.81 The Protek Duo® dual-lumen cannula (CardiacAssist, Pittsburgh, PA, USA) contains two lumens, with one serving as an inflow tract positioned in the right atrium, and the other serving as the outflow tract positioned in the pulmonary artery. Similarly to the Impella RP, an oxygenator can be spliced into the circuit, and such device configuration is known as the oxy-RVAD (oxygenator in right ventricular assist circuit).82 Much of the evidence pertaining to the use of these two centrifugal flow pumps comes from case reports and case series. The THRIVE study (TandemHeart in right ventricular support) retrospectively evaluated 46 patients who received TH-RVAD for acute RV failure.83 TH-RVAD acutely improved haemodynamics such as MAP, right atrial pressure, pulmonary artery systolic pressure and cardiac index, and in-hospital mortality was found to be 57%.83
The use of the Protek Duo has been commonly described in RV failure following LVAD implantation and also pulmonary hypertensive crisis.84 VA-ECMO and IABP have also been used for right-sided support and also in biventricular failure (described above). Unlike LVAD, experience related to RVADs is very limited and warrants future investigational trials, development of algorithms for risk stratification, device selection and weaning protocols.
Short-term MCS devices in structural heart valve interventions
Structural heart disease remains an uncommon cause of CS and remains an underexplored avenue for MCS device utilization, with much of the current evidence in this area based on small observational studies.
IABP remains the most used MCS device for structural valvular interventions, especially transcatheter aortic valve implantation.85 Nevertheless, IABP use in aortic stenosis can be associated with worsening aortic regurgitation. Eliaz et al. reported a case series on the use of IABP during implantation of the MitraClip® device (Abbott Laboratories, Chicago, IL, USA).86 The authors reported that IABP led to better leaflet coaptation and increase in coaptation surface that allowed better leaflet grasping, providing durable reduction of mitral regurgitation and avoidance of mitral stenosis. The Impella has been commercially used to provide mechanical support in aortic stenosis, especially with concomitant LV dysfunction and coronary artery disease.87 A fundamental concern with the insertion of the Impella is worsening stenosis due to narrowing of the orifice by the catheter, and also risk of cerebral embolic events due to the interaction between the calcified valve and the inflow cannula. The use of the TandemHeart is limited due to the need for transseptal puncture, increased time for circulatory support and limited experience. ECMO is used in at least 4% of patients undergoing transcatheter aortic valve implantation, primarily in emergent situations as bail out for procedural complications.88 Advantages of VA-ECMO include quick bedside access, high-flow circuit and ability to provide concomitant pulmonary support. Unlike the Impella or the TandemHeart, there is no need for transseptal puncture or crossing of the aortic valve.
Long-term mechanical circulatory
support devices
Selection of long-term mechanical
circulatory support
As with CS, classification systems such as the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) have further delineated patients with end-stage refractory heart failure and New York Heart Association class III and class IV who are likely to benefit from durable MCS implantation (Table 4).89 Many of the available long-term MCS devices were initially developed for acute profiles 1 and 2, but are now increasingly used in patients with profiles 3 and 4.90 Most of the currently available durable MCS devices are all intracorporeal in location. The pump technology has evolved from first-generation pulsatile flow devices to second-generation continuous axial flow with contact bearings/seals, to third-generation centrifugal continuous flow devices without contact bearings/seals. Currently, more than 90% of MCS devices in the USA are continuous flow devices, while the remaining 10% are pulsatile total artificial heart (TAH) (Table 5).4


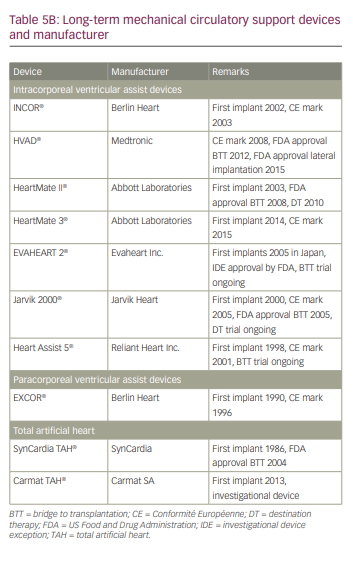
In the USA, long-term MCS device implantation is currently available for two payer-approved conditions: bridge to transplantation and destination therapy. The decision on which approach to take is mostly driven by the insurance payer in the USA. The US Centers for Medicare and Medicaid Services approved indications for LVAD implant are presented in Table 6. As with temporary MCS devices, it is important to know the contraindications when selecting a durable MCS device (Table 7).


First-generation left ventricular assist devices
First-generation LVADs are pulsatile volume displacement pumps, which include the HeartMate XVE® (Abbott Laboratories , Chicago, IL, USA), Thoratec Paracorporeal Ventricular Assist Device (PVAD®; Abbott Laboratories, Chicago, IL, USA, formerly Thoratec Corp.) and Novacor® (Baxter Healthcare Corp., Novacor Division, Oakland, CA, USA). Only the Thoratec PVAD is commercially available now, although it is rarely used due to the availability of newer generation devices. Reports from the INTERMACS registry show that the use of these devices has declined significantly over the past decade.90
Second-generation left ventricular assist devices
Axial flow pumps such as the HeartMate II, Jarvik 2000 and the MicroMed DeBakey VAD® (MicroMed Technologies, Woodlands, TX, USA) have a rotary pump suspended by mechanical bearings. The speed of the pump is proportional to the pressure gradient generated between the inflow and outflow cannulas. However, the mechanical bearings make them more prone to thrombosis and haemolysis. As such, aspirin in addition to systemic anticoagulation is used with all axial flow pumps. In the recent INTERMACS registry annual report, these devices accounted for 78% of the total durable MCS devices implanted between 2006 and 2017.90
HeartMate II
The HeartMate II® (Abbott Laboratories, Chicago, IL, USA) is a continuous, axial flow pump with a titanium-coated rotor that can generate flows of up to 10 L/min at pump speeds of 6,000–10,000 rpm. It received FDA approval for bridge to transplantation in April 2008 and for destination therapy in January 2010. It was compared against the first-generation HeartMate XVE in the HeartMate II trial in patients deemed ineligible for cardiac transplantation.91 The results showed that use of the continuous axial flow pump was associated with higher survival rates and lower adverse events compared with pulsatile flow devices. The ROADMAP (Risk assessment and comparative effectiveness of left ventricular assist device [LVAD] and medical management; ClinicalTrials.gov identifier: NCT01452802) study was a prospective non-randomized trial that compared HeartMate II with optimal medical therapy.92 Survival at 1 year and 2 years was greater with the LVAD than with optimal medical therapy. Pump thrombosis remains a major concern with this device due to direct contact between mechanical bearing and blood. This was subsequently evaluated in the PREVENT (Prevention of HeartMate II pump thrombosis; ClinicalTrials.gov identifier: NCT02158403) trial, which showed that the incidence of pump thrombosis at 3 months and 6 months was 2.9% and 4.8%, respectively. However, adherence to therapeutic anticoagulation recommendations was associated with significantly lower risk of pump thrombosis.93
Jarvik 2000
The Jarvik 2000® (Jarvik Heart, New York, NY, USA) is a continuous axial flow pump that sits completely within the LV cavity and can generate flows of up to 7 L/min. It weighs about 85 g and measures 5.5 cm × 2.4 cm.
The INCOR® LVAD (Berlin Heart AG, Berlin, Germany) is another axial flow pump that is marketed in Europe but not available in the USA.
Third-generation left ventricular assist devices
The third-generation centrifugal pumps such as the HeartMate 3 and HeartWare Ventricular Assist Device work in similar fashion to the CentriMag, although they are smaller in size. The continuous flow is generated by a centrifugal rotor with a single moving part that is fully levitated in an electromagnetic field. The blades generate a ‘coanda’ effect by swirling the blood against housing, with minimal shear stress compared with an axial pump.94 Other differences between axial and centrifugal flow pumps include:
1. sensitivity to afterload: centrifugal flow pumps are more afterload sensitive (i.e. greater flow reduction with increasing afterload);
2. suction: centrifugal flow pumps are less prone to suction events than axial flow pumps; and
3. pulsatility: centrifugal flow pumps generate greater pulsatility than axial flow pumps.
HeartMate 3
The HeartMate 3® (Abbott Laboratories, Chicago, IL, USA) is an intrapericardial, fully magnetically levitated centrifugal flow pump that can generate flows of up to 5 L/min. The device is CE marked for use in Europe and was approved by the FDA in 2018. The HeartMate 3 was evaluated in a prospective single-arm, non-randomized study, the HM3 CE mark trial (HeartMate 3™ CE mark clinical investigation plan; ClinicalTrials.gov identifier: NCT02170363).95 The mortality at 1 year was 18%, there was no evidence of pump thrombosis or malfunction, and quality of life and functional capacity improved over time. The HeartMate 3 was compared against the HeartMate II in the MOMENTUM III trial (Multicenter study of MagLev technology in patients undergoing mechanical circulatory support therapy with HeartMate 3; Clinical Trials.gov identifier: NCT02224755).96,97 Although there was no difference in mortality between the two devices, the HeartMate 3 had superior mechanical performance due to fewer pump malfunction events requiring repeat surgeries. The HeartMate 3 was also associated with lower risk of pump thrombosis compared with the HeartMate II (1.4% versus 13.2%). Furthermore, superior treatment effects were observed with the HeartMate 3, irrespective of whether the indication was bridge to transplantation or destination therapy.98 A pilot trial, MAGNETUM 1 (Minimal anticoagulation evaluation to augment hemocompatibility; ClinicalTrials.gov identifier: NCT03078374), evaluated the safety and feasibility of targeting lower international normalized ratio in the range of 1.5–1.9 in patients with the HeartMate 3 due to its improved safety profile.99 The primary endpoint of survival free of pump thrombosis, disabling stroke and major bleeding at 6 months was achieved in 93% of study participants (n=15). Some complications associated with use include outflow graft twisting, RV failure, aortic insufficiency and infections. The FDA released a field safety notice in April 2018 following multiple reports of outflow graft twisting with the HeartMate 3 and finally, issued a class I recall of this device in May 2018.100
HeartWare Ventricular Assist Device
The HeartWare Ventricular Assist Device (HVAD®; Medtronic, Minneapolis, MN, USA) is a continuous-flow, third-generation centrifugal pump approved by the FDA for bridge to transplantation in November 2012 and for destination therapy in September 2017. Similarly to the HeartMate 3, it can generate flows of up to 10 L/min. It was compared with the HeartMate II in the ENDURANCE trial (The HeartWare™ ventricular assist system as destination therapy of advanced heart failure: the ENDURANCE trial; ClinicalTrials.gov identifier: NCT01166347), which enrolled 446 patients deemed ineligible for cardiac transplantation.101 Survival rates were comparable between the two groups, although HeartWare had a higher incidence of stroke, RV failure and sepsis. In the HeartWare ADVANCE (Evaluation of the HeartWare left ventricular assist device for the treatment of advanced heart failure; ClinicalTrials.gov identifier: NCT00751972) bridge to transplantation trial that enrolled 140 patients with HeartWare who were compared with a control group of 499 patients implanted with a commercially available pump and awaiting cardiac transplantation enrolled in the INTERMACS registry, the primary outcome of survival to 180 days without a change in device, or transplantation, was similar between the two groups (90.7% versus 90.1%).102 Compared with other commercially available devices, the HeartWare device was noted to have higher incidence of neurological events and mortality in observational studies.103 Thus, this device was taken off the market in June 2021 and is no longer being manufactured.104
Biventricular support devices
Total artificial heart
The SynCardia TAH® (SynCardia, Tucson, AZ, USA), consists of two artificial ventricles, each with a stroke volume of 70 mL, and can generate flows of up to 9.5 L/min. The device was originally developed from the Jarvik 7 TAH. The device is recommended for patients with a body surface area >1.7 m2 and a thoracic diameter ≥10 cm.105 The device was compared in a non-randomized study that included 81 patients who received TAH and 35 control patients who were not able to receive TAH although they met the entry criteria.106 The 1-year survival rate among TAH recipients was 70% compared with 31% in the control group. Common complications included infection, bleeding, neurological adverse events, device malfunction and multiorgan failure. SynCardia TAH is approved as a bridge to transplantation and is currently being investigated as a destination therapy in adults who are not eligible for heart transplantation (ClinicalTrials.gov identifier: NCT02232659).107 A new portable TAH has received the CE mark and is currently undergoing trials in the USA.108,109
Conclusion
CS is a life-threatening condition due to cardiac pump dysfunction and is associated with high mortality. Despite technological advancements and rigorous research on CS, managing patients who are resistant to conventional pharmacological therapies remains a challenge. This review has provided insights into available MCS devices, their mechanisms of action, selection of appropriate patients for MCS use, data relating to available MCS devices and the need for early use of MCS. More RCTs investigating MCS use in various clinical scenarios of CS are needed to guide optimal use of these devices in the future.












