Introduction: 3D models derived from late gadolinium-enhanced cardiac magnetic resonance imaging (LGE-CMR) can reduce ventricular tachycardia (VT) ablation procedure times and improve outcomes. Clinician-led manual segmentation is time and resource intensive. However, neural networks can be trained to identify and segment cardiac structures, mimicking clinician decisions, whilst operating at a fraction of the time with little to no inter-operator variability. Most published segmentation models are limited by small training datasets, restricted labelling outputs (usually left ventricle only) and often lack correlated clinical data. We aimed to develop an artificial intelligence biventricular geometry and scar segmentation model addressing these limitations.
Methods: Clinically indicated LGE-CMR in those with ischaemic scar were retrospectively identified, following ethical approval. Clinical data was extracted from healthcare records. Ground-truth manual CMR segmentation of seven labels was performed – left ventricular (LV) myocardium, LV blood pool, LV scar, aorta, LV papillary muscles, right ventricular (RV) myocardium and RV blood pool. A neural network was developed utilising transformers, zero-centre normalization, average of dice loss and weighted cross entropy. LGECMR datasets were separated into a 70:10:20 split for training, validation and testing respectively. Results were assessed using Dice co-efficient and average symmetric surface distance (ASSD).
Results: Four hundred LGE-CMR from 2017 to 2021 were labelled, 362 obtained from a 1.5T Philips Ingenia and 38 from a 1.5T Siemens Aera. Median slice number per scan was 11, with 10 mm slice thickness. This resulted in a total of 4,515 slices, of which 2,373 (52.6%) were labelled ‘optimal’ quality and only 144 (3.19%) ‘uninterpretable’. All images were 2D and did not utilize compressed sense (or equivalent). Median age was 66 years (56–74), with a male predominance (80.5%). Cardiac devices consisting of implantable cardioverter defibrillators (7) and pacemakers (5) were present in 12 (3%) patients. One hundred and fourteen (28.5%) had diabetes and 153 (38.3%) had previous cardiac stenting. A history of previous, confirmed, ventricular arrhythmia was
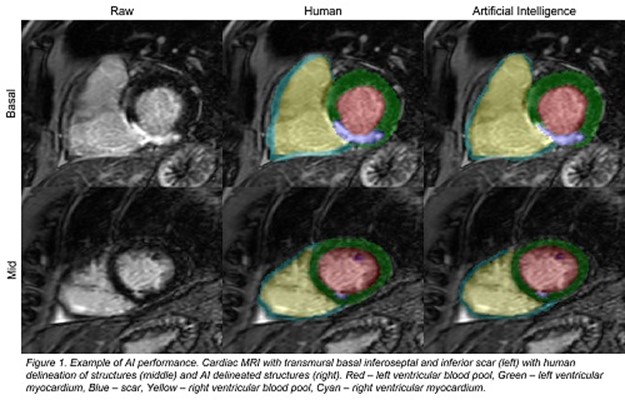
present in 15 (3.8%). The overall mean Dice was 0.667 and mean ASSD was 1.52 mm. The left ventricular blood pool had the highest mean dice (0.888) and second lowest mean ASSD (0.818 mm). Scar had a mean dice of 0.439 and a mean ASSD of 2.416 mm.
Conclusion: This model trained on a large 2D, disease-specific dataset demonstrates scar labelling performance similar to previously published models with the advantage of multiple structure segmentation and a geometrical 3D output that can be imported into electro-anatomical mapping systems as a navigational aide. Furthermore, the dataset included varied ‘real-world’ image quality in the most common cause of ventricular scar and included patients with implanted cardiac devices, often excluded in other published models. Further development and training of the model using higher resolution LGE-CMR studies is currently underway (NotIs CMR study, IRAS:298151), recruiting prospective IHD scans from multiple CMR manufacturers using different acquisition settings to improve future generalizability and applicability in the clinical setting. Concurrent paired clinical data is being collected for future use in risk prediction modelling. ❑
Figure 1: Example of AI performance