Introduction: The rate of cardiac implantable electronic device (CIED) placement is increasing. CIED placement comes with complications. CIED infection is a major complication increasing both morbidity and mortality. International guidelines do not recommend the local use of specific antimicrobial agents during implantation because adequate scientific studies supporting their use are not available or not supportive. In this prospective observational study, the infectious complications that occurred after CIED placement performed following a new periprocedural prevention protocol with taurolidine were recorded.
Materials and methods: All participants undergoing CIED placement (including generator substitution, revision with the aim to upgrade or downgrade, early revision, i.e. any procedure accessing the pocket) between 1 January 2020 and 30 November 2022 at 4 European hospitals were consecutively included in the study. Procedures and not the participants were the datasets. During each procedure all hardware (leads, suture sheaths, pulse generator) was treated with an antimicrobial solution containing taurolidine (TauroPace) and the pocket irrigated with the same product. Primary endpoint was major CIED infection occurring within 3 and 12 months after the procedure, while secondary endpoints were all grade adverse events (related to the antimicrobial solution used, the CIED system or the procedure) and all-cause mortality.
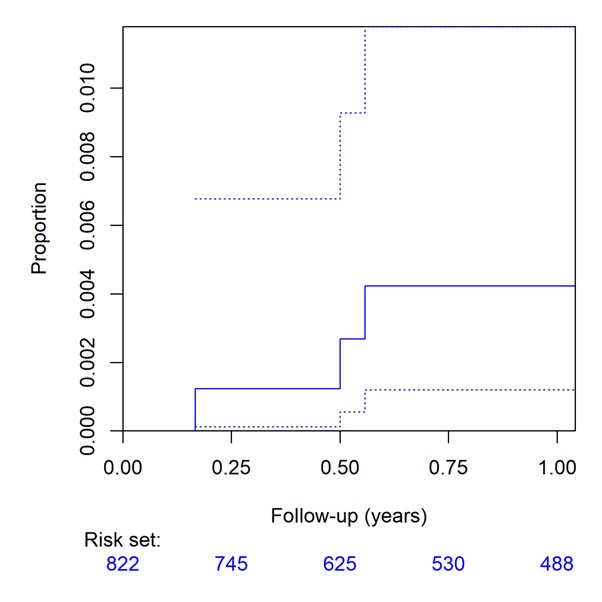
Results: Adjunct taurolidine was used in 822 procedures which entered final analyses. Three major CIED infections could be observed during 12 months. Kaplan-Meier estimated event rates were 1/799 (0.125%) at 3 months [0.003–0.695%] and 3/588 (0.51%) at 12 months [0.105–1.48%], respectively. Two major CIED infections were pocket infections with Kaplan-Meier estimated event rates of 0/799 (0%) at 3 months [0–0.461%] and 2/588 (0.34%) at 12 months [0.041–1.220%], respectively.
Discussion: Infections are an important and clinically significant complication associated with the placement of a CIED.1 Current epidemiological data indicate an increasing number of infections with suspicion of underdetection.2 Numerous treatments have been proposed to meet this problem with inconsistent results.
Taurolidine is a versatile agent. It is the main active ingredient of an antimicrobial wash designed for disinfecting CIED hardware and the surgical site (pocket). Its active metabolites prevent pathogens from adhering to surfaces, destroying them, preventing biofilm formation and neutralising endotoxins and exotoxins. There is good evidence of the molecule’s efficacy at different galenic concentrations in preventing foreign body infections in renal replacement therapy, parenteral nutrition and cytostatic treatment.
Our temporal analysis suggests the clinical utility of taurolidine use for the prevention of CIED infection, particularly pocket infection in a cohort of high-risk patients, showing lower event rates than those reported in the literature.
Conclusions: Taurolidine is safe and effective for the prevention of infectious complications related to the placement of any CIED. ❑
Figure 1: Cumulative incidence curves for CIED pocket infection (top) and major CIED infection (bottom) by cohort (solid lines) with pointwise confidence intervals (dashed lines)