Pericarditis is the most common form of pericardial disease, with an incidence of 27.7 cases per 100,000 population per year.1 Approximately 15–30% of patients with acute pericarditis will have a first recurrence within 18 months despite optimal medical therapy. Half of these patients will fail to achieve complete remission and will continue to have recurrent flares of symptoms.2,3 Recurrent pericarditis is associated with significant morbidity and adversely affects quality of life.4 This condition is identified by a recurrent flare of symptoms 4–6 weeks after the index episode of pericarditis. The mainstay of treatment includes non-steroidal anti-inflammatory drugs (NSAIDs), colchicine and corticosteroids. Corticosteroid-sparing regimens are often required due to the side effects related to chronic use.5 The interleukin (IL)-1 pathway has emerged as a promising target for the treatment of these patients.3 The objective of this review is to describe the mechanism of action and the anti-inflammatory effect of rilonacept, a novel IL-1 inhibitor, in the treatment of recurrent pericarditis.
Pathophysiology of recurrent pericarditis and the interleukin-1 pathway
The underlying pathophysiology of recurrent pericarditis remains poorly understood. Complex interplay between environmental triggers, coupled with a heightened immunologic response in susceptible hosts with compromised immune systems or genetic susceptibility, are described as determining factors.6,7 Adaptive immunity was considered the key pathway in the development of recurrent pericarditis through several inappropriate response mechanisms, such as activation of dormant viral particles, inflammation-related transformation of self-antigens into foreign antigens, molecular mimicry and the development of auto antibodies (anti-heart, anti-nuclear and anti-intercalated discs) found in elevated concentrations in pericardial fluid.8–12
Contemporary studies have emphasized the role of innate immunity in the disease process by identifying similar signal pathways between the idiopathic form of recurrent pericarditis and autoinflammatory conditions, such as familial Mediterranean fever and tumour necrosis factor alpha (TNF-α)-associated periodic syndrome.13 These conditions share a common pathway with activation of a cytosolic macromolecule ‘inflammasome’, which recognizes molecular damage and pathogens to elicit downstream inflammatory response.6 Inflammasomes consist of three main components:
- adaptor protein ASC;
- procaspase-1 enzyme; and
- sensor molecule, a nucleotide-binding oligomerization domain-like receptor (NOD) pyrin domain-containing 3 (NLRP3).
NLRP3 is stimulated by microbes through pathogen-associated molecular patterns, non-infectious triggers through damage-associated molecular patterns (DAMPs) and/or by TNF-α.Activation of NLRP3 inflammasone stimulates thepro-inflammatory nuclear factor-kappa B (NF-kB) transcription complex leading to the release of IL-1.6,14 IL-1 is a potent pro-inflammatory cytokine that leads to recurrent pericardial inflammation primarily through the action of the subtypes IL-1α and IL-1β. The former is released from stress cells and injured pericardial cells activating DAMPs in inflammatory cells and promoting the transcription of IL-1β. Moreover, this inflammatory milieu enhances pericardial inflammation via further activation of NLRP3 amplifying IL-1 production. Finally, IL-1β also activates cyclooxygenases, the classical pathway of inflammation, which is targeted by NSAIDs and corticosteroid anti-inflammatory drugs (Figure 1).6,15–17
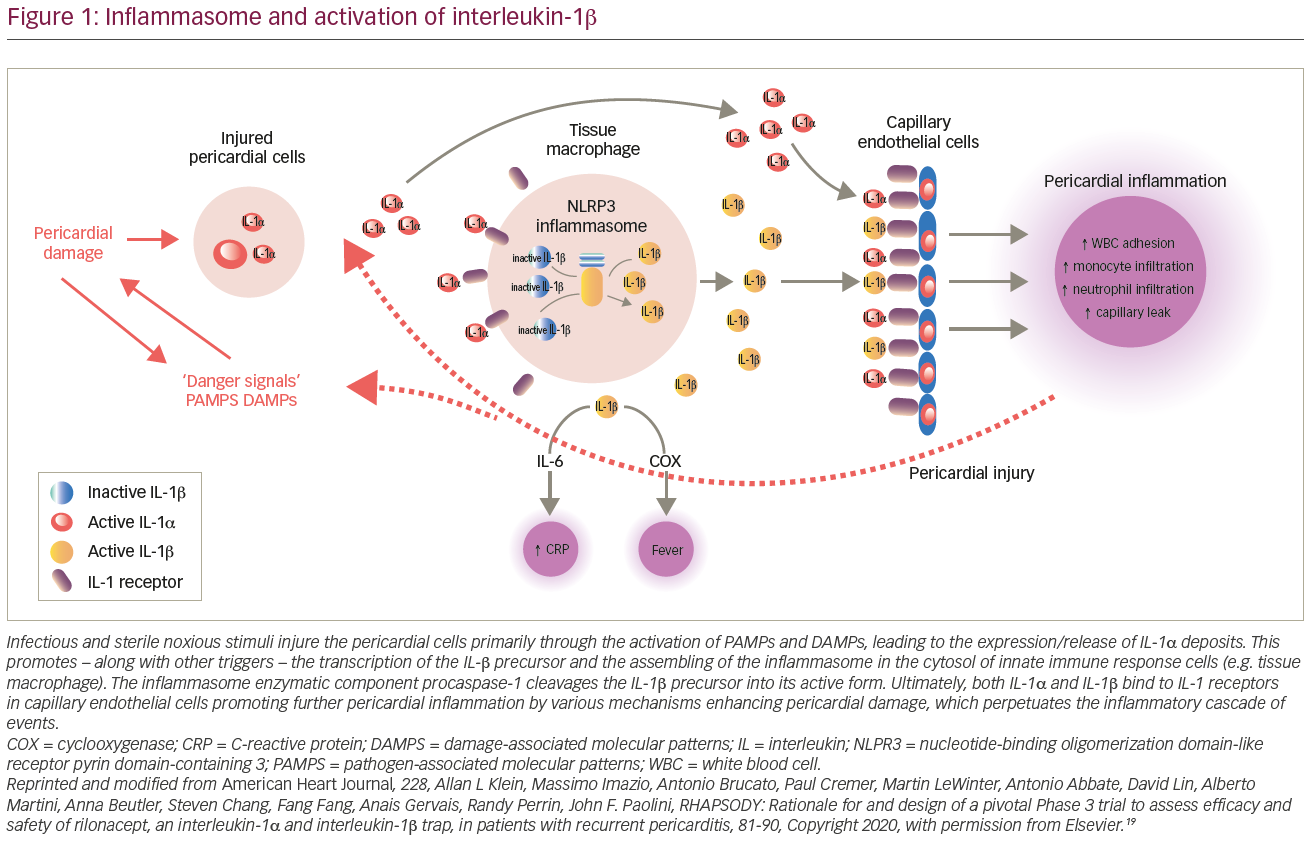
Recurrent pericarditis is the culmination of several dysregulated autoimmune and autoinflammatory processes. Although these two pathways may be present simultaneously, based on the predominance of one of them, patients can manifest a non-inflammatory phenotype (e.g. underlying autoimmune disease) or an autoinflammatory phenotype.18
Biochemical structure and mechanism of action of rilonacept
Rilonacept is a dimeric fusion protein composed of three structures: the ligand binding-domain of the human IL-1 receptor, IL-1 receptor accessory protein, and the Fc portion of the human immunoglobulin (Ig)G1.19 Circulating rilonacept effectively functions as an ‘IL-1 trap’, binding to the circulating IL-1α and IL-1β, and with less affinity, to the IL-1 receptor antagonist that naturally mitigates their inflammatory response. This interaction blocks the engagement of IL-1β to the cell surface receptors, inhibiting the downstream inflammatory cascade.17,20,21
Clinical studies of rilonacept
In 2008, rilonacept received US Food and Drug Administration (FDA) approval for the treatment of the rare genetic condition cryopyrin-associated periodic syndrome.21 Two major studies were published in 2020 and 2021, the RHAPSODY phase II and RHAPSODY phase III trials, which demonstrated the efficacy of rilonacept for the management of patients with recurrent pericarditis.22,23 RHAPSODY phase II was an open label, single-arm, five-part pilot study designed to evaluate the efficacy and safety of rilonacept in patients with recurrent pericarditis. The trial enrolled subjects from January 2018 to May 2019 at nine sites across the USA. Eligible candidates included adults (18–75 years) or children (6–<18 years) with at least two recurrences presenting with either active disease (symptoms and either C-reactive protein [CRP] >1 mg/dL or delayed gadolinium enhancement on magnetic resonance imaging [MRI]) or without active disease attributable to corticosteroid dependence. Based on the clinical presentation and whether the aetiology was deemed idiopathic or post-pericardiotomy syndrome, five different subgroups (‘parts’) were defined. Patients were allowed to receive concomitant treatment with colchicine, NSAIDs or corticosteroids in any combination. Active treatment consisted of a loading dose of 320 mg rilonacept (KPL-914), administered via subcutaneous (SC) injection on day 0, followed by 160 mg SC weekly for five additional doses. An optional 18-week treatment-extension period was established, with the possibility of being cautiously weaned off concomitant anti-inflammatory medications.
The primary efficacy outcome for patients with active disease was based on patient-reported pericarditis pain using an 11-point pain numerical rating scale (NRS) and CRP change. For those without active disease attributable to corticosteroid dependence, the primary efficacy outcome was reactivation of the disease after corticosteroid taper.22
A total of 49 patients were screened and 25 adults were enrolled, of which, 23 entered in the extended period. The mean age was 42.8 years (range 26–62 years), 60% were female, the majority of patients were white, and 80% were taking two or more anti-inflammatory medications. For symptomatic patients with active disease and elevated CRP >1 mg/dL regardless of its aetiology (n=13), pain was reduced by an average of 4 points on the 11-point NRS scale, and CRP normalized in all patients in a median time of 9 days (Figure 2). For the remaining three symptomatic patients with active disease and delayed gadolinium enhancement on cardiac MRI, there was a decrease in NRS score, and CRP remained normal throughout the study.22

The key finding was a reduction in the incidence of recurrent pericarditis episodes per year, from 3.9 (standard deviation [SD] 3.66) to 0.18 (SD 0.62) in patients with idiopathic recurrent pericarditis and active disease, as demonstrated by elevated CRP, and 0.0 for the remaining study parts. Of the 13 patients who completed the study and were receiving corticosteroids at baseline, 11 (84.6%) discontinued corticosteroids and the remaining 2 had the dose reduced without recurrence of symptoms.22
This breakthrough was followed by the results from the recently published phase III RHAPSODY study, a double-blinded, placebo-controlled, multi-centre, randomized-withdrawal trial in patients with symptomatic recurrent pericarditis (at least two recurrences) and elevated CRP (>1 mg/dL) despite conventional therapy.23 The trial consisted of four periods: the first ‘screening period’ lasted 4 weeks to assess eligibility. The second ‘run-in period’ lasted 12 weeks, where the medication was administered (loading dose 320 mg or 4.4 mg/kg if <18 years of age; followed by weekly 160 mg or 2.2 mg/kg if <18 years of age), concomitant anti-inflammatory therapy was weaned and rilonacept was continued as monotherapy (the last 2 weeks). Patients that met a prespecified clinical response (no symptoms and CRP <0.5 mg/dL) entered the ‘randomization-withdrawal period’, which consisted of 1:1 randomization to weekly rilonacept versus placebo. Finally, after the trial closure by the accrual of a prespecified number of recurrences, eligible patients from the run-in and randomization-withdrawal periods were transitioned to the last period, ‘long-term extension’, when patients were offered up to 24 months of open-label rilonacept.23
The primary efficacy endpoint was the development of recurrent episodes, and secondary efficacy endpoints included assessment of clinical response at 16 weeks, time to normalization of CRP and the time by which patients discontinued standard therapy and were receiving rilonacept monotherapy. A total of 86 patients were enrolled; 61 had completed the run-in period and undergone randomization before enrolment was stopped. The mean age was 44.7 years, 57% were female, 85% were idiopathic and the remaining 15% were secondary to post-cardiac injury. The median duration of rilonacept treatment, including the run-in period, was 9 months (3–14 months). There was a rapid resolution of pericarditis pain (median of 5 days), normalization of CRP (7 days) (Figure 3A) and successful withdrawal of corticosteroids in the treatment group. The rilonacept group had significantly fewer recurrences compared with the placebo group (7% versus 74%; hazard ratio 0.04; 95% confidence interval 0.01–0.18; p<0.001 by the log-rank test) (Figure 3B). Interestingly, the median time from initiation of rilonacept to tapering and discontinuing of standard therapy was 7.9 weeks. Moreover, there was no re-introduction of corticosteroids or pericarditis recurrence. These results suggest that rilonacept is an effective and safe therapy for recurrent pericarditis. It may be used as a monotherapy, and may help to discontinue standard therapy. The significant efficacy of rilonacept as monotherapy supports the hypothesis that an autoinflammatory IL-1-mediated process may be the key step in the pathophysiology of recurrent pericarditis in patients with elevated systemic inflammation.23

Safety profile
The RHAPSODY phase II preliminary study demonstrated good safety and tolerability of rilonacept.22 In this study, 92% of the adverse events were mild to moderate in severity – the most common being transient injection site reactions (15/25 patients, 60%), and the remaining included nasopharyngitis (4/25, 16%), arthralgia (3/25, 12%) and diarrhoea (3/25, 12%). None of these events led to drug discontinuation and all were treated with conservative measures. No major laboratory abnormalities were reported; however, there was an increase in non-fasting total cholesterol, low-density lipoprotein cholesterol and triglycerides, but these did not require therapy initiation.22 However, there were no data on cardiovascular outcomes of rilonacept-related hyperlipidaemia. It is recommended to monitor lipid profiles in these patients and treat accordingly based on each patient’s cardiovascular risk.
The favourable safety profile and tolerability demonstrated in RHAPSODY phase II was corroborated in RHAPSODY phase III.22,23 The most common adverse reactions in individuals who received rilonacept were injection site reactions (34%) and upper respiratory tract infections (23%). The majority of adverse events were transient and mild to moderate in severity. Four patients had adverse events that warranted drug discontinuation, including alopecia, extrinsic allergic alveolitis, systemic allergic reaction and erythema, which were all reported during the run-in period.23 At this time, it remains unknown if slow-tapering protocols can ameliorate the inflated numbers of recurrences that occur after medication withdrawal, as was demonstrated with anakinra, another IL-1 inhibitor.24 However, rilonacept has a longer half-life when compared with anakinra (7 days versus 4–6 hours, respectively); therefore, it may not need slow tapering.21,25
The safety data of rilonacept in pregnancy is limited to animal studies; these show a possible harmful effect on embryo–foetal development.25 Therefore, rilonacept is classified as category C and should only be used when the benefit to the patient outweighs the risk of foetal toxicity. Otherwise, it should be discontinued before pregnancy. It is also unknown whether rilonacept is secreted during lactation; however, since anakinra (IL-1β recombinant receptor antagonist) was found in human milk, rilonacept should be used with caution in this population and preferably discontinued during lactation.26
There are no studies available regarding drug interactions with rilonacept. Hypothetically, since elevated IL-1 levels could inhibit the production of CYP450 enzymes, rilonacept may be able to restore the production of the CYP450 enzymes. This will require concentration and therapeutic effect monitoring, in addition to dose adjustments of medications that serve as substrate for this enzyme, such as warfarin. The concomitant use of anti-IL-1 drugs and TNF-α blocking agents is not recommended due to the presumed increased risk of immunosuppression and serious infections. All recommended vaccinations should be completed before the initiation of rilonacept, as IL-1 blockade may suppress the protective immune response to the vaccine. It is recommended that rilonacept is not used concomitantly with live vaccines.25
Although rilonacept could potentially attenuate the formation of antibodies elicited by the messenger RNA COVID-19 vaccines, patients with pericarditis treated with immunosuppressive agents were not included in the trials of vaccines; however, this potential remains only speculative.27,28 On the other hand, anti-IL-1 agents appear to be well tolerated in the context of COVID-19 infection, and it seems to be prudent to continue treatment, if necessary, for symptom control.29
Administration protocol
Rilonacept can be administered by the patient or caregiver as a SC injection in the abdomen, thigh or upper arm, after proper instruction and training on the aseptic technique of injection.25 The therapy is initiated in adults (18 years and older) with a loading dose of 320 mg, followed by a maintenance dose of 160 mg injection once per week, similar to the phase III trial protocol, in addition to conventional background medications, such as NSAIDs, colchicine and/or corticosteroids.22,23 In paediatric patients (12–17 years), the loading dose is 4.4 mg/kg, up to a maximum of 320 mg, delivered as one or two SC injections (maximum single injection volume, 2 mL), followed by a maintenance dose of 2.2 mg/kg once per week, up tp a maximum of 160 mg (maximum single injection volume, 2 mL).25
The optimal length of therapy with rilonacept remains to be defined. Results from the RHAPSODY phase III long-term extension arm are highly anticipated and hope to clarify the issue of duration. In addition, real-world data from the planned Registry of the Natural History of Recurrent Pericarditis in Pediatric and Adult Patients (RESONANCE; ClinicalTrials.gov Identifier: NCT04687358) is intended to collect substantial data to help inform treatment duration.30 The steady trough concentrations of rilonacept are not significantly influenced by gender, body weight or age, and no dose adjustment is required in patients with liver and renal impairment.31,32 Rilonacept should be stored in the refrigerator at a temperature between 2°C and 8°C (36–46°F) and kept inside the carton to protect it from light. The efficacy and safety of this potential monotherapy may reduce the overall cost burden of recurrent hospital admissions and prolonged hospital stay on the healthcare system at large. On 18 March 2021, rilonacept received FDA approval for treatment for recurrent pericarditis.33
Conclusions
A new era is starting, with rilonacept as a promising therapy for patients with recurrent pericarditis. Results from the RHAPSODY trial have endorsed rilonacept as an efficacious and safe immunomodulator with potential use as monotherapy (Table 1). These results have shifted the paradigm of the understanding and management of this disease, further emphasizing the primary role of the autoinflammatory response in recurrent pericarditis. However, more studies are needed to evaluate the appropriate duration of therapy, the need for tapering protocols, the utility of rilonacept in the various stages of pericardial inflammation and to assess its safety in specific populations, such as children and pregnant women. In addition, the efficacy of rilonacept is hypothesis-generating for the future evaluation of biomarkers of the autoinflammatory pathway that may better identify suitable candidates for treatment with novel targeted immunomodulator therapy.