Introduction: Medical therapy for heart failure with reduced ejection fraction (HfrEF) improves quality of life, reduces heart failure hospitalization and mortality, and reduces the risk of ventricular arrhythmias. International guidelines recommend four key drug classes for all patients with HfrEF. However, many patients do not receive them. Implementing clinical pathways to increase access to HfrEF medical therapy is therefore a high priority.
In the UK, most patients with implantable cardiac devices are followedup in a device clinic led by cardiac physiologists. Many of these patients also have HfrEF but do not have ongoing review in a heart failure clinic if they are clinically stable. As a result, they may not be prescribed current guideline-directed medical therapy. A new clinical pathway was therefore created to identify patients with HfrEF under follow-up in the device clinic, and then refer them to a combined device and heart failure clinic to optimise their medical therapy.
Methods: The pathway was implemented in a single UK tertiary cardiac centre. The cardiac physiology team were trained to screen patients attending device follow-up clinics for HfrEF, through review of medical records and cardiac imaging. All patients with HfrEF, who were not under the active care of a heart failure team, were referred to a combined device and heart failure clinic.
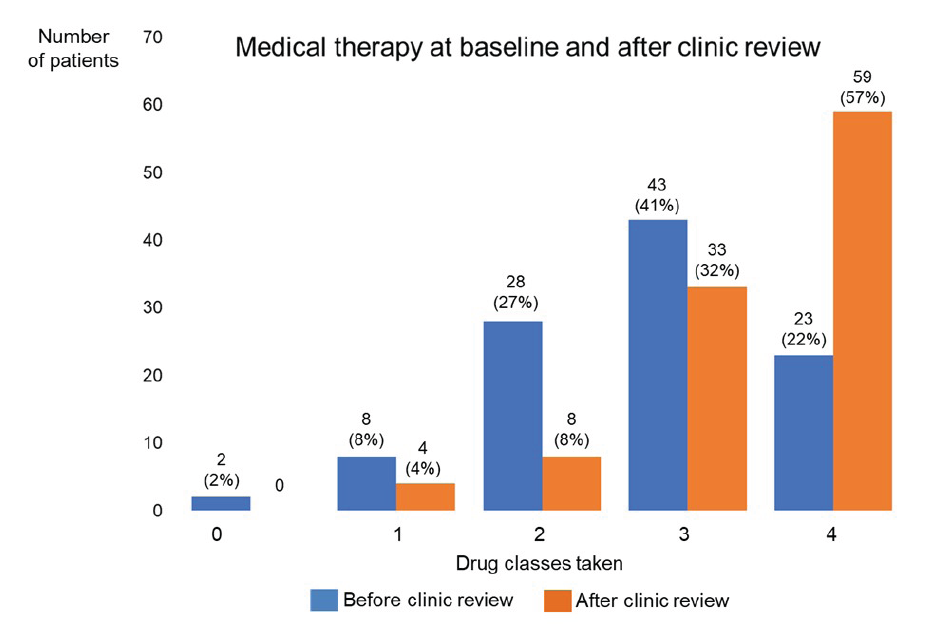
Results: One hundred and forty-seven patients were referred to the new combined clinic from August 2022 over a 7-month period. One hundred and forty-one out of 147 (96%) patients attended; 104/141 (74%) had HfrEF and 37/141 (26%) had either HfmrEF or HfpEF. Of the 104 patients with HfrEF, mean age was 71 years (range 29–94) and 76% were male. At baseline, 8/104 (8%) were in NYHA class I, 56/104 (54%) class II, 40/104 (38%) class III and 0/104 in class IV. Forty-four out of 104 (42%) had a CRT-D, 26/104 (25%) had a CRT-P, 17/104 (17%) had a single or dual chamber ICD, 9/104 (9%) had a single or dual chamber pacemaker and 8/104 (8%) who were referred from other sources did not have a device. At baseline, only 23/104 (22%) patients with HfrEF were prescribed all 4 drug classes. Following combined clinic review, after an average of 1.8 appointments, 59/104 (57%) patients were prescribed these four drug classes (see Figure 1). In addition, drug doses prescribed at baseline were up-titrated in 39/104 (38%).
Conclusion: Screening all patients attending the cardiac physiologist-led device follow-up clinic was an effective way to identify those with HFrEF who were not under the care of a heart failure team. In this population, medical therapy at baseline was not optimal. Following combined device and heart failure clinic review, the number of patients taking all 4 guidelinedirected drugs for HFrEF increased markedly from 22% to 57%. Joint working between heart failure services and implantable cardiac device services can therefore both identify patients with HFrEF who are under-treated and then significantly improve their medical therapy. ❑
Figure 1: Medical heart failure therapy drug classes at baseline and following combined device and heart failure clinic review