Introduction: The restriction of activation mapping to the ventricular surface of contemporary mapping systems often leads to failure to correctly identify the true site of origin (SOO) of intramural and/or sub-epicardial Vas and lower procedural success. Electromechanical wave imaging (EWI) is a non-invasive echocardiography-based high frame rate technology that offers direct transmural activation mapping and may allow to overcome the limitations of established mapping approaches.
Methodology: Patients with complex ventricular ectopy (VE) and clinical indication for VE-ablation were recruited to undergo preprocedural EWI to identify the SOO and validate against contact mapping. The study has received a favourable opinion of the REC (Ref 14/LO/0360).
EWI was performed using a P4-2 phased array transducer (Phillips ATL) with a VantageTM 256 research ultrasound system (Verasonic Inc.). An US sequence composed of a 2 second B mode followed by 4 seconds 2,000 FPS single diverging wave was acquired in 6 apical views and simultaneous ECG acquisition. The displacement estimation was performed on the RF signal from each probe element with 1D axial cross-correlation followed by a least square estimator. Local electromechanical activation was defined as the time point of the downward zero crossing (ZC) on the incremental axial strain. For each view, 200–250 ZCs on manually selected strain curves were annotated on a segmented B-mode mask. Activation times in milliseconds were colour-coded to generate isochronic maps.
Site of earliest activation in contact mapping and successful ablation was defined as ground truth for VE SOO. Epicardial origin was confirmed by direct epicardial mapping or suspected if, endocardially, no pre-QRS activation and/or broad early area with r wave in unipolar EGMs was observed. Septal intramural SOO was confirmed by venous mapping or suspected based on combined right and left ventricular activation mapping features. Papillary muscle origin was defined based on anatomical location (supported by co-registered CT model), typical pleomorphic VE morphologies with subtle changes and multiple exit sites (no intramural mapping data to confirm precise SOO available for these sites).
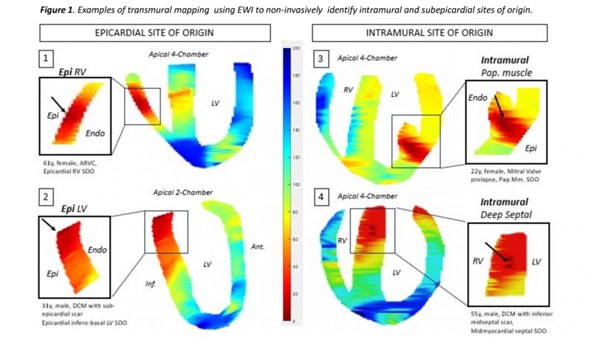
Results: Eleven patients with VE were enrolled (54.5% male, age 39 ± 17.4 years, 64% structural heart disease with LV EF 43 ± 16% and 50% LGE+ in MRI). Total VE burden was 26.2 ± 9.2%. Seven patients had one prior ablation attempt. Contact mapping identified the SOO as subepicardial in 3 (ARVC with RV epicardial SOO, LV summit, DCM with epicardial infero-basal LV SOO), deep intramural septal in 2, LV papillary muscle in 4, right aortic cusp in 1 and endocardial mid RV free wall in 1. EWI correctly identified all (sub)epicardial (3/3) and intramural septal (2/2) SOOs. Three papillary muscle Ves were mapped to the base/intramural segment of the papillary muscle and 1 to the midmyocardium in the adjacent LV wall segment. The aortic cusp VE was mapped to the LVOT. The patient with endo RV VE had insufficient Ves for EWI mapping at two attempts. Representative examples of EWI localization maps are shown in Figure 1.
Conclusion: EWI successfully identified intramural and subepicardial origin of focal ventricular arrhythmias independent of presence of scar. This promising, non-invasive and radiation-free echocardiography-based mapping modality has the potential to guide preprocedural planning including requirement for epicardial access and/or advanced ablation setup and technology (e.g. bipolar, ethanol). ❑
Figure 1: Examples of transmural mapping using EWI to non-invasively identify intramural and subepicardial sites of origin