Athletes represent the fittest individuals in our society, yet paradoxically carry an increased risk of sudden cardiac death (SCD) when compared to sedentary individuals with the same cardiac disease.1,2 Two recent studies have provided a realistic prevalence of young SCD in both the general population and in elite athletes. A population-based study from Australia and New Zealand showed the incidence of young SCD in the general population to be 1.3/100,000 among individuals aged 1–35, whilst a recent study of cardiac screening in young adolescent football players in the UK showed a much higher incidence of SCD of 6.8/100,000 in this athletic population.3,4 Indeed, SCD is the most common non-traumatic cause of mortality in athletes.5 Studies have shown that sports activity in adolescents can trigger an increased rate of sudden death, especially in the presence of underlying structural heart disease.2 Up to 66% of SCD cases in athletes have been shown to be potentially identifiable on pre-participation electrocardiogram (ECG), including inherited, electrical or structural abnormalities.5–7 In addition, there are a number of physiological adaptations to the ECG in high-level athletes, which can be misinterpreted as pathology and lead to a number of expensive and unnecessary further investigations in the athlete with a risk of increased stress, anxiety and cost to the health system.8
Whether the ECG is performed for screening or for diagnosis, the ability of the reviewing physician to identify normal athletic adaptation is critical. In the last 10 years, a number of studies have highlighted some key electrical features of normal physiological adaptation in a number of different ethnic groups and have led to multiple revisions of the original 2010 recommendations for ECG interpretation in athletes.9–15 This has led to the recently updated ‘International recommendations for ECG interpretation in athletes’, published in 2017.5 These recommendations are more specific for identifying athletes with disease, and resulted in a reduction in the number of athletes with an abnormal ECG – from 22% to 3% compared with the 2010 European Society of Cardiology (ESC) criteria.16 Currently, the ESC, as well as a number of leading sporting organisations, including the International Olympic Committee, recommend screening of elite athletes for underlying cardiovascular disease using a combination of history, examination and pre-participation ECG.17 Therefore, it is essential that the reviewing clinician has expertise in identifying athletes with high-risk ECG markers, and differentiating normal athletic adaptation from pathology.
Normal athletic adaptation of the electrocardiogram
There are a number of physiological adaptations that occur in the heart as a consequence of exercising intensively for >4 hours/week including increased vagal tone and chamber dilatation. These adaptations lead to specific associated changes on the ECG that can be considered normal and do not warrant further investigations (Table 1).5 Sinus bradycardia is observed in the majority of athletes; however, a heart rate <35 bpm is rare, except in endurance athletes.18

Sinus arrhythmia is also common, occurring in up to 70% of athletes, whilst first-degree atrioventricular (AV) block is present in approximately 10–15%.12,13,19 The PR interval in an athletic individual is not considered to be pathologically abnormal until it is >400 ms.5 Junctional rhythm, Mobitz type 1 second-degree AV block (Wenkebach) and sinus pauses (up to 2 seconds) are seen in a proportion of athletes, especially during sleep.20 Other changes attributable to high vagal tone include ectopic atrial rhythm and wandering atrial pacemaker.21 Higher degree of AV block, including prolonged sinus pauses >3 seconds during waking hours, Mobitz type 2 second-degree AV block and third-degree AV block, are exceptionally rare in athletes and warrant further investigation.5
Repolarisation changes are also commonly seen on the athlete’s ECG. Early repolarisation patterns with J-point elevation, saddle-backed ST-segment elevation, notching of the J point and slurring of the downstroke of the S-wave, are common in athletes and present in up to 5% white athletes and up to 40% of black athletes.13
Despite some evidence of increased early repolarisation in survivors of cardiac arrest with idiopathic ventricular fibrillation,22 there are currently no data to support increased risk of SCD in athletes with early repolarisation in isolation, and therefore should be considered benign in the absence of other clinical markers of pathology.5,21 There are also well-described repolarisation abnormalities that have been observed in up to 13% of black athletes with a pattern of convex (upsloping) ST elevation followed by T-wave inversion V1–V4, which is considered normal variation in black athletes.5,13
Voltage criteria for left and right ventricular hypertrophy in the absence of other abnormalities (such as T-wave inversion) are common in athletes and do not warrant further investigation. Large voltages are partly due to physiological increases in ventricular cavity size and mass, but are also commonly due to less distance between the heart and the ECG electrode in slim males. Voltage criteria for left and right ventricular hypertrophy are observed in 50–60% and 13% of male athletes, respectively.5,23
Right ventricular dilatation is a common manifestation of physiological adaptation to exercise.24,25 This is believed to cause increased conduction time through the His–Purkinje fibres and manifests as incomplete right bundle branch block (RBBB) on ECG, which is seen in up to 30% of athletes. This ECG, showing QRS <120 ms and rSR’ in V1, is considered a normal ECG pattern in athletes and does not warrant further investigation in an asymptomatic athlete.12,15,19 Some athletes may demonstrate incomplete RBBB with J-point elevation and mildly ascending ST segments in the right praecordial leads (type 2 Brugada pattern).21 Repeating the ECG with the leads V1 and V2 placed higher in the second or third to exclude a spontaneous type 1 Brugada pattern (which is not a normal variant), is recommended.26
Normal T-wave inversion patterns
T-wave inversion in the general population and in white athletes is considered normal in V1–V2 and in leads III or aVF. In white athletes, anterior T-wave inversion beyond lead V2 is rare, particularly in men.27,28 In the black athletic population anterior T-wave inversion is acceptable in V1–V4, especially when preceded by J-point elevation and upsloping ST segment which has been shown to be present in almost 13% of the black athlete popuation.13 Anterior T-wave inversion is also more prevalent in endurance athletes compared with non-endurance athletes. T-wave inversion in leads V1–V2 is identified in 10% of endurance athletes and may extend to V3 in approximately 4%.29 In young individuals, anterior T-wave inversion to lead V3 is known as the ‘juvenile pattern’ and is present in up to 3% of young athletes and non-athletes aged <14 years old. However, this pattern rarely persists after age 16 in adolescent athletes.12,30
Normal QT interval in athletes
The normal QT interval in the general population is <440 ms in men and <460 ms in women; however, athletes may exhibit a longer QT interval due to the effect of increased vagal tone or delayed repolarisation secondary to increased left ventricular mass.31 It is important to accurately measure the QT interval and exclude U waves, especially in leads V2–V4. The QT interval is best measured in leads II and V5. The QT should be corrected for heart rate using Bazett’s formula (QTc = QT/√RR) accepting that it is less accurate at the extremes of heart rate (Bazett’s formula tends to under-correct at high heart rates >120 bpm and over-correct at low heart rates <40 bpm).21 In cases of sinus arrhythmia it is best to use the averages of the QT and the RR interval, as this approach has been shown to have very high accuracy.32 The acceptable upper limits for a normal QT interval are ≤470 ms in males and ≤480 ms in females. With regards to the shortest QTc duration in athletes, a cut-off of >320 ms is accepted as the lower range of normal as 99.9% of athletes have a QTc in this range.33
Abnormal electrocardiogram in athletes
Underlying structural and electrical disease in athletes can lead to ECG changes that are beyond what would be acceptable for their degree of athletic activity. These changes should act as ‘red flags’ for the reviewing physician and initiate consideration of further cardiac investigations including echocardiography, Holter monitor testing, exercise tolerance testing and cardiac magnetic resonance (CMR) imaging (Table 2).

Abnormal T-wave inversion
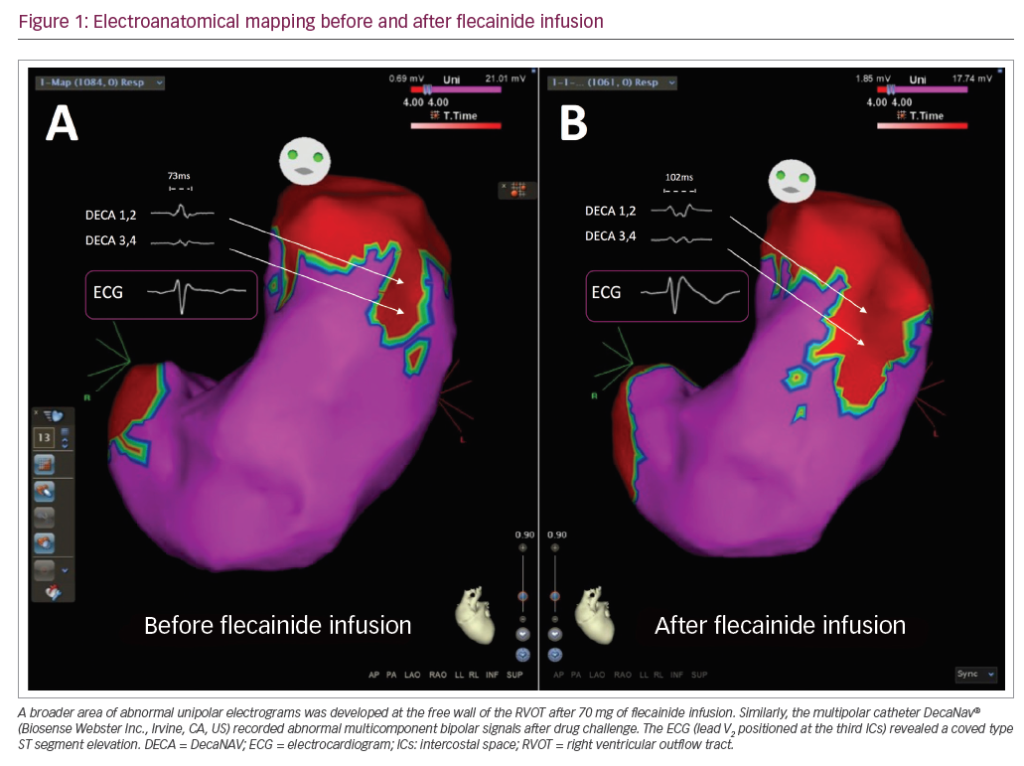
T-wave inversion is a well-recognised manifestation in individuals with cardiomyopathies. Although some patterns of T-wave inversion are acceptable in young adolescents and in all black athletes, the presence of this anomaly should trigger a search for other clues in the history or the ECG to determine an underlying abnormality.13,34,35 Anterior T-wave inversion beyond V2 in adult non-black athletes is considered abnormal and should warrant further investigation to exclude arrhythmogenic (right ventricular) cardiomyopathy (ARVC).5 Other co-existing electrical anomalies that may favour the diagnosis include the presence of an epsilon wave (a distinct low amplitude signal between the end of the QRS complex and the onset of T-wave in leads V1–V3), delayed upstroke to the S-wave in V2, small limb lead complexes (QRS <0.5 mV) and ventricular premature beats.5,36 T-wave inversion in the lateral leads should raise the possibility of an underlying cardiomyopathy and warrant further investigation with echocardiogram ± CMR imaging with late gadolinium enhancement to assess for myocardial fibrosis (Figure 1A and B). Although T-wave inversion in the lateral leads has been documented in 4% of black male athletes and is unlikely to represent heart disease in all individuals, there are documented cases of black athletes with the abnormality that have either been diagnosed with cardiomyopathy or had an aborted sudden cardiac arrest.37 Therefore, all athletes with lateral T-wave inversion require further assessment irrespective of ethnicity.

The significance of inferior T-wave inversion is uncertain. This repolarisation anomaly is detected in <0.3% of white athletes but present in 6% of black athletes. We have yet to diagnose a cardiomyopathy in a black athlete with isolated T-wave inversion; therefore, whilst it may be worthwhile to perform comprehensive investigations in white athletes with this abnormality, we would not consider any further investigations beyond echocardiogram in asymptomatic black athletes with isolated inferior T-wave inversion.
ST-segment depression
ST depression ≥0.5 mm (0.05 mV) in ≥2 leads is abnormal; it is not a usual manifestation of athletic adaptation but is common in patients with cardiomyopathy and therefore should be investigated with an echocardiogram and consideration for CMR imaging in some cases.5
Pathological Q-waves
The criteria for pathological Q-waves in athletes has been updated in the international recommendations and is now defined as a Q/R ratio of ≥0.25 or ≥40 ms in duration in two or more leads. Pathological Q-waves may indicate underlying cardiomyopathy, infiltrative disease, accessory pathways or myocardial infarction.5 Occasionally V1–V2 may manifest pathological Q-waves as a pseudo-septal infarct pattern if the leads are placed too high relative to the cardiac position.34 Q-waves in isolation, particularly in lead aVL, are relatively common in young athletes and should not be considered pathological unless they extend beyond the parameters described above.21
Conduction delay patterns
High vagal tone and tendency to bradycardia is a known manifestation of athletic adaptation and may be normal in some athletes; however, in athletes with profound first-degree AV block (PR ≥400 ms) and profound sinus bradycardia (heart rate <30 bpm or sinus pause >3 secs), further investigation to exclude chronotropic incompetence (such as exercising on the spot) is indicated. High-grade AV block (Mobitz type 2 second-degree AV block or complete heart block) are abnormal and require further investigation with echocardiogram, Holter monitor, exercise tolerance testing and referral to an electrophysiologist.5 Complete left bundle branch block (LBBB) pattern is rare in athletes and common in patients with cardiomyopathy and therefore always warrants further investigation with echocardiogram and CMR imaging with perfusion study. Similarly, athletes with severe non-specific interventricular conduction delay (QRS duration ≥140 ms) should be investigated.
Ventricular pre-excitation
Ventricular pre-excitation due to an underlying accessory pathway leads to the Wolff–Parkinson–White pattern on the ECG with short PR interval (<120 ms), a delta wave (slurring of initial QRS) and QRS duration >120 ms, occurs in up to 1 in 250 athletes (Figure 2).38,39 An exercise tolerance test should be the first investigation in the athlete, and evidence of abrupt complete loss of accessory pathway with higher heart rates is indicative of a lower risk accessory pathway.40 An echocardiogram is recommended to exclude any associated structural disease such as Ebstein’s anomaly or cardiomyopathies. Electrophysiological studies and ablation of the accessory pathway should be considered, particularly in competitive athletes involved in moderate to high-intensity sports due to risk of a rapidly conducted atrial fibrillation across an accessory pathway, leading to ventricular fibrillation.5

Prolonged QT interval
Prolongation of the QT interval on ECG is the hallmark of congenital long QT syndrome (LQTS), an inherited arrhythmia syndrome with an estimated prevalence of 1 in 2,000 and a predisposition to
ventricular arrhythmias and potential SCD.41 Prolongation of the QTc ≥470 ms in males and ≥480 ms in females is considered abnormal (Figure 3). Abnormal QT prolongation in the absence of QT-prolonging medications or electrolyte abnormalities warrants further investigation with repeat ECGs and consideration for exercise testing, family screening and potentially genetic testing through a physician with specialised expertise in this area.5,41 Features suggestive of LQTS in an asymptomatic athlete include relevant family history, QT >500 ms and paradoxical lengthening of the QTc in the fourth minute of recovery of exercise tolerance test.41,42

Type 1 Brugada pattern
Brugada syndrome is an inherited arrhythmia syndrome with a prevalence of 1 in 2,000 characterised by a cove-typed ST elevation in the right praecordial leads (Figure 4).41 The coved-type ST elevation in the Brugada ECG pattern is identified by a broad r’ and a downsloping ST-segment (between the J-point and 80 ms after the J-point) compared to an upsloping ST-segment in the early repolarisation patterns of an athlete. In an athlete with a borderline Brugada ECG pattern, the ECG should be repeated with high leads (V1–V2 placed in second and third intercostal spaces). The type 1 Brugada ECG pattern in an athlete should always be further investigated further for risk of SCD, and first-degree family members should also be reviewed and screened.5,41

Multiple premature ventricular contractions or ventricular arrhythmias
It is considered unusual for an athlete to have >2 premature ventricular contractions (PVCs) on baseline ECG and usually warrants more prolonged ECG monitoring with a Holter monitor to assess the arrhythmic burden.43 Athletes generally reveal extrasystoles arising from the right (LBBB with inferior axis) or left (RBBB with inferior axis) ventricular outflow tracts or the left fascicles (relatively narrow RBBB with left or right axis) which resolve with increasing heart rate during exercise; such patterns of PVCs are considered benign.44 Other patterns of extrasystoles such as LBBB with superior or intermediate axis or atypical RBBB with QRS ≥130 ms are unusual and may be associated with underlying myocardial disease. The burden of PVCs can be an important marker of underlying structural heart disease. In athletes with ≥2,000 PVCs per 24 hours, up to 30% have been found to have underlying structural heart disease.43,45 In such athletes, or those with increasing ectopy with exercise testing, further investigation is indicated including echocardiogram, CMR imaging and potentially electrophysiological testing including electroanatomic mapping when appropriate or occasionally endomyocardial biopsy.5 There is emerging evidence that PVCs conducting with broad RBBB and a superior axis that increase during exercise may represent left ventricular scar.46 Identification of subepicardial or midmyocardial scar on CMR imaging, particularly in a striae pattern, is associated with ventricular arrhythmias and SCD in athletes.47 Ventricular couplets, triplets and non-sustained ventricular tachycardia always requires further investigation to evaluate for structural heart disease, ARVC or other cardiomyopathies.5 If underlying genetic heart disease is suspected, then genetic testing can be useful in some circumstances (Figure 5).48

Conclusion
There are a number of electrical patterns that can develop as part of the athletic adaptation of the heart. However, there are also some ECG abnormalities that should be considered as red flags to the reviewing physician of underlying structural, arrhythmic or inherited heart disease. It is important to ensure ECGs of athletic individuals are reviewed and interpreted by a physician who is trained in differentiating physiology from pathology in this special population.