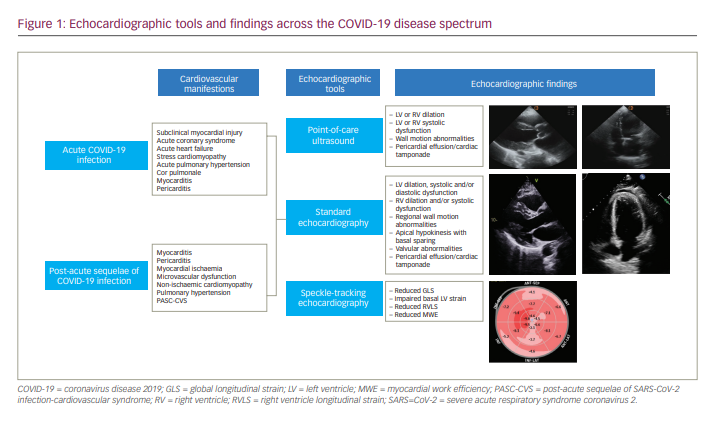
As of the publication of this article, the coronavirus disease 2019 (COVID-19) infection has affected over 400 million people around the world and caused over 6 million deaths.1 Although COVID-19 infection predominantly affects the respiratory system, studies have described a wide spectrum of cardiovascular manifestations, including asymptomatic myocardial injury, myocardial infarction and myocarditis.2 Echocardiography is an easily accessible, quick and cost-effective imaging modality that has been widely used in patients with COVID-19 for the evaluation of cardiac structure and function.2–5 More recently, speckle-tracking echocardiography (STE) has been used to detect subclinical myocardial dysfunction in patients with COVID-19.3,6–8 One study reported that echocardiographic findings affected changes in management in 33% of COVID-19 cases, suggesting the important role played by echocardiography in guiding therapeutic approaches.9 Furthermore, certain echocardiographic abnormalities have been shown to correlate with biomarkers and mortality in patients with COVID-19, highlighting the additional prognostic value of echocardiography.6,10 With increasing reports of cardiovascular sequelae of COVID-19, the role of echocardiography has expanded to diagnosing and managing patients who have recovered from acute COVID-19 infection. This article aims to describe the echocardiographic tools currently available and to summarize the echocardiographic findings across the COVID-19 disease spectrum and their associations with biomarkers and clinical outcomes.
Echocardiographic tools and recent advances
Echocardiography is a commonly used imaging tool for the evaluation of cardiac structure and function in patients with COVID-19 and suspected myocardial injury. When images are technically difficult to obtain, ultrasonic enhancing agents (echocardiographic contrast) are used to improve diagnostic yield.11 The major benefits of echocardiography are easy and quick performance at the bedside, wide accessibility, lack of radiation and low cost. Some of the limitations of echocardiography include variability in image quality based on the patient’s body habitus or the sonographer performing the study and infectious exposure to the sonographers.12
During the COVID-19 pandemic, cardiac point-of-care ultrasound (POCUS) gained popularity, especially in emergency and critical care settings, as it provides real-time bedside information in the acute setting.3,13–15 Although it is more limited compared with standard two-dimensional echocardiography due to lower resolution and sometimes lack of Doppler features, POCUS can help to assess the basic structure and function of the left ventricle (LV) and right ventricle (RV) and to detect wall motion abnormalities with lower equipment burden and viral transmission risk (Figure 1).3,13,14 Therefore, POCUS has played an important role in diagnosing, monitoring and risk-stratifying patients with COVID-19.
Several technological advances have improved the diagnostic capabilities of echocardiography. STE is a newer modality that is used in conjunction with conventional echocardiography and tracks unique speckle pathways to calculate the percentage change in myocardial deformation throughout the cardiac cycle.6,7,16 STE provides a precise estimation of a regional and global contractile function known as global longitudinal strain (GLS). STE has been shown to have higher sensitivity compared with standard echocardiography in identifying myocardial dysfunction in influenza myocarditis, immune checkpoint inhibitor-related myocarditis and dilated cardiomyopathy.17–19 Another novel tool is global myocardial work efficiency (MWE) assessment, which uses STE-driven strain indexed to systolic blood pressure and allows a more load-independent measure of LV function.6,8,20 MWE assessment is highly reproducible, and some studies suggest that it is even superior to GLS in detecting subclinical myocardial dysfunction and predicting adverse events.6,19,21
Guidelines and indications for echocardiography during the coronavirus disease 2019 pandemic
At the start of the COVID-19 pandemic, the American Society of Echocardiography (ASE) recommended careful triage of patients undergoing echocardiography in order to reduce the risk of virus transmission.22 The ASE advised postponing elective cases and performing problem-focused, limited echocardiography for urgent or emergency indications only if the examination was to offer essential clinical information in the short term.22 As the number of new COVID-19 cases down-trended, and many facilities resumed their echocardiographic services for non-urgent and elective cases, the ASE recommended careful monitoring of COVID-19 case trends and available institutional resources, focused protocols and limited examinations for follow-up studies, as well as considering preprocedural COVID-19 testing in patients with suspicion of infection at routine screening (e.g. symptoms recorded on questionnaire, high temperature on testing).23
With regard to specific indications for echocardiography, the American College of Cardiology (ACC) Clinical Guidance for COVID-19, released in 2020, recommended echocardiography for patients with COVID-19 who demonstrated heart failure, arrhythmia, electrocardiography changes or cardiomegaly.24 In patients with cardiac sequelae of COVID-19 after recovery from initial illness, the ACC recommends an echocardiogram as part of the initial triad testing along with electrocardiography and troponin.25 It is important to highlight that, when there are concerning electrocardiography abnormalities, elevated troponin or ventricular wall motion abnormalities, other diagnostic tests, imaging tools and invasive procedures should be considered. Specifically, when myocarditis is suspected, the ACC recommends cardiac magnetic resonance imaging in haemodynamically stable patients and right heart catheterization and/or endomyocardial biopsy in patients with shock, sustained ventricular arrhythmias and/or advanced atrioventricular blocks.25
Echocardiographic findings in acute coronavirus disease 2019 infection
A wide spectrum of echocardiographic manifestations has been reported in patients with COVID-19, ranging from subclinical myocardial dysfunction to severe LV or RV dysfunction (Figure 1).26 Table 1 summarizes the findings of key echocardiography studies conducted during the COVID-19 pandemic. It is important to note that the majority of these studies do not report baseline echocardiography data prior to acute COVID-19 infection and that most include patients with cardiovascular diseases at baseline, including heart failure. However, studies that exclude patients with previous abnormal echocardiography or cardiovascular comorbidities have found similar echocardiographic abnormalities during acute COVID-19 infection. The demographic data, comorbidities and severity of COVID-19 infection of the patients included in the studies are detailed in Table 1.6,8,9,27–33




Left ventricle abnormalities
One of the largest available studies on echocardiographic findings in COVID-19 showed that, among 1,216 patients (14% with ischaemic heart disease, 9% with heart failure and 7% with valvular disease at baseline) across 69 countries, LV abnormalities were present in 39% of cases during acute COVID-19 infection.9 Impaired left ventricular ejection fraction (LVEF) has been reported in up to 35% of patients with COVID-19.10,28,34–36 However, the specific causative aetiology of reported LV abnormalities are not identifiable, as most COVID-19 studies have not included prior (pre-infection) echocardiography data. Furthermore, variation in case definitions for acute myocarditis or coronary syndrome during COVID-19 infection has made confirming these diagnoses challenging, and definitions are often not included at all.
Many studies also suggest that LV systolic dysfunction is not a common manifestation in patients with COVID-19.9,34,35 For example, Szekely et al. found that, among 100 patients hospitalized for COVID-19 infection, 28% of whom had a high COVID-19 Modified Early Warning Score, LV systolic dysfunction was seen in only 10% of patients, despite including 16% of patients with ischaemic heart disease and 7% with congestive heart failure at baseline.30 In another study that excluded patients with previously abnormal echocardiography, Mahmoud-Elsayed et al. reported that LV function was rarely impaired and more often normal (in 41% of cases) or hyperdynamic (in 48% of cases) in acute COVID-19 infection.29 Furthermore, 82% of these patients required mechanical ventilation, and 38% died, showing that LV systolic function can be preserved even in severe cases of COVID-19 infection.
Data on LV dilation and diastolic dysfunction in patients with COVID-19 are more limited. One study found LV dilation in 4% of cases,29 and a few studies have noted LV diastolic dysfunction in 6–49% of patients with COVID-19.27,30,36 However, it is often difficult to discern the acuity of diastolic dysfunction, given the high prevalence of comorbidities, including hypertension, in patients with COVID-19. Additionally, stress cardiomyopathy, with characteristic transient apical hypokinesis with basal sparing, has been observed in up to 6% of cases, mostly in older women.9,28,37,38
Right ventricle abnormalities
RV dysfunction has been reported in patients with COVID-19 and may be secondary to severe respiratory disease, acute pulmonary hypertension, cor pulmonale, pulmonary thromboembolism, right-sided myocardial infarction or focal myocarditis.39,40 In the study by Szekely et al., in which 61% of patients had moderate disease requiring non-invasive oxygen therapy and 10% of patients needed mechanical ventilation, the authors found that RV dilation with or without RV dysfunction was the most common echocardiographic abnormality in COVID-19.30 RV dilation was noted in 39% of patients, and abnormal parameters of RV systolic function, namely reduced RV fractional area change, systolic tricuspid lateral velocity, Tei index and tricuspid annular plane systolic excursion (TAPSE), were found in 28%, 25%, 20% and 14% of patients, respectively.30 Other studies have similarly noted high incidences in both RV dilation (in up to 49% of patients) and RV dysfunction (in up to 40% of patients) in COVID-19 (Table 1).9,27–29,31 However, it is important to recognize that studies by van den Heuvel et al. and Kim et al. found RV dysfunction in only 10% and 8% of patients, respectively.36,41 This discrepancy is likely to be the reflection of different patient cohorts with various disease severity, as none of the patients in the study by van den Heuvel et al. were on mechanical ventilation at the time of the echocardiographic examination.
Strain and myocardial work assessment
With the use of STE, there is increasing evidence that focal abnormalities may be present even when the global LVEF is preserved on standard echocardiography.6,42 Minhas et al. found abnormal GLS (absolute value <16%) in 47% of patients hospitalized with COVID-19, 57% of whom developed acute respiratory distress syndrome (ARDS), and 19% of whom died.6 Furthermore, the authors noted that, among patients with normal LVEF, 36% had abnormal GLS.6 In this study, 72% of patients had pre-existing hypertension, 15% had coronary artery disease and 15% had heart failure, and baseline STE data prior to COVID-19 infection were not available. However, even when patients with abnormal baseline echocardiography (LVEF <50%, wall motion abnormalities, severe valvular disease) and cardiovascular comorbidities (coronary artery disease, atrial fibrillation, pulmonary hypertension, renal failure) were excluded, Baycan et al. found the mean LV GLS to be abnormal (-16.8 ± 2.5%) in patients with COVID-19, while mean LVEF was preserved (60.3 ± 4.6%).8 Similarly, for the RV, patients with COVID-19 have been shown to have abnormal RV longitudinal strain (RVLS).8,33 One study of 214 patients, including those with hypertension (57%), heart failure (10%) and ischaemic heart disease (16%) at baseline, found that the mean RVLS was abnormal at -19.8 ± 5.9%.33 Together, these findings suggest that subclinical myocardial dysfunction is probably common in COVID-19 and is underestimated by standard echocardiography. However, a major limitation of these studies is the lack of pre-infection echocardiography data, which restricts the ability to make any conclusions regarding the causation between COVID-19 infection and myocardial injury. Prospective studies and control groups will be fundamental in future investigations.
In addition to abnormal GLS, STE studies have identified specific regional strain patterns in patients with COVID-19. Both Goerlich et al. and Stöbe et al. found reduced basal LV strain in more than 50% of patients hospitalized with COVID-19.16,42 This abnormal LV deformation in the basal segment, which has been observed in infiltrative cardiomyopathies such as Anderson–Fabry disease and different forms of myocarditis, including influenza myocarditis, is thought to reflect the susceptibility of certain myocardial regions to inflammatory or systemic stressors.16,43,44
Although data regarding MWE assessment in patients with COVID-19 are extremely limited, Minhas et al. reported that 79% of patients hospitalized with acute COVID-19 undergoing STE had abnormal MWE (<95%), and that 74% of patients with preserved LVEF had abnormal MWE.6 Therefore, in some cases, abnormal MWE may be an earlier or the only indicator of COVID-19-related myocardial dysfunction.
Other echocardiographic findings in acute coronavirus disease 2019 infection
Regional wall motion abnormalities have been reported in up to 14% of patients with COVID-19.10,28,41 Although it is difficult to discern the acuity of valvular abnormalities, aortic regurgitation, aortic stenosis, mitral regurgitation and tricuspid regurgitation have been reported in patients with COVID-19 (Table 1).27,28,30–32 Pericardial effusion, which can be secondary to pericarditis or perimyocarditis, has been reported in up to 7% of patients with COVID-19.27–29,45 Cardiac tamponade is rare but has been reported in several case reports46–48 and in 1% of patients in one study.9
The correlation of echocardiographic findings with biomarkers and clinical outcomes in coronavirus disease 2019
Several studies have reported correlations of echocardiographic findings with biomarkers or disease severity in COVID-19. Jain et al. found an inverse relationship between high sensitivity (hs)-cardiac troponin T and LVEF (ρ=-0.34, p=0.006).28 A lower absolute value of LV GLS has also been associated with higher hs-troponin I (r=0.633, p<0.001) and D-dimer (r=0.577, p<0.001).8 For diastolic dysfunction, Lairez et al. reported significantly lower lateral mitral annual diastolic velocity in patients with COVID-19 with elevated troponin levels compared with those with normal troponin levels (p=0.03).49 Another study found a higher E/e’ ratio in patients with severe ARDS compared with those with normal oxygenation (p=0.03).50
With regard to RV abnormalities, RV systolic dysfunction (defined by fractional area change) has been associated with elevated D-dimer (ρ=-0.34, p=0.003) and C-reactive protein (r=-0.23, p=0.045)29 in patients with COVID-19. A lower absolute value of RVLS has also been associated with higher hs-troponin I (r=0.608, p<0.001) and D-dimer (r=0.620, p<0.002).8 Additionally, patients with ARDS have been shown to have a significantly lower absolute value of RVLS compared with those without ARDS (p<0.001).32
There is increasing evidence that echocardiographic findings not only correlate with biomarkers but also correlate with mortality. One study found that myocardial injury and echocardiographic abnormalities together were associated with higher in-hospital mortality, whereas myocardial injury alone was not, highlighting the prognostic value of echocardiography.27 In patients with COVID-19, low LVEF has been associated with mortality (odds ratio [OR]=12.19; 95% confidence interval [CI]: 2.87–51.83; p=0.001).31 An elevated E/e’ ratio has also been associated with death (hazard ratio [HR]=1.08; 95% CI: 1.01–1.2; p=0.03), suggesting that LV diastolic dysfunction, in addition to LV systolic dysfunction, may be a sign of poor clinical outcome.30 Both studies that include patients with cardiovascular diseases at baseline and those that exclude patients with abnormal echocardiography, known cardiomyopathy or ischaemic heart disease have shown that TAPSE is significantly reduced in non-survivors compared with survivors32,33,51 and that RV dysfunction portends a poor prognosis in COVID-19 infection.31,32,41,52
STE-derived strain data have also been shown to be of prognostic benefit in COVID-19. Patients who died have been shown to have significantly worse LV GLS than those who survived.53 Baycan et al. more specifically reported that the absolute values of LV GLS <15.20% (HR=8.34; 95% CI: 2.78–79.35; p<0.001) and RVLS <18.4% (OR=6.22; 95% CI: 1.51–25.67; p=0.01) were associated with increased mortality.8 Furthermore, Lassen et al. found that the risk of mortality increased with worsening LV GLS (HR=1.20; 95% CI: 1.07–1.35; p=0.002, per 1% decrease in absolute value) and worsening RVLS (HR=1.64; 95% CI: 1.02–2.66; p=0.043, per 1% decrease in absolute value), indicating that the degree of myocardial dysfunction also offers prognostic value.33
Finally, MWE was found to be 2.04% lower per higher tertile of interleukin-6 level (p=0.021), and higher MWE was associated with lower in-hospital mortality (adjusted OR=0.87; 95% CI: 0.78–0.97; p=0.009).6 These results suggest that MWE may correlate with the degree of systemic inflammation and also predict clinical outcomes in COVID-19.6
Echocardiographic findings in patients recovered from coronavirus disease 2019 infection
Although follow-up data are limited, significant diastolic abnormalities have been reported at 6-month follow-up in patients with elevated troponin levels during index hospitalization for COVID-19 infection.54 STE studies have also shown that abnormal GLS, and specifically reduced strain in the basal segments of the LV, can persist for 1 to 2 months after recovery from COVID-19 infection.42,55,56 Interestingly, Lassen et al. showed that, while TAPSE and RVLS improved 2 months after COVID-19 infection, LV GLS did not, although both LV and RV functions were impaired at follow-up compared with matched controls.57 More studies are needed to see whether LV GLS eventually improves or if LV GLS abnormalities persist even after a longer follow-up duration. Furthermore, there is evidence that even asymptomatic patients who were not hospitalized for COVID-19 infection had a significantly lower absolute value of LV GLS at follow-up (median: 23 [interquartile range: 11–89] days) compared with healthy controls.58 This study excluded patients with COVID-19 who had abnormal electrocardiography, LVEF <50%, severe valvular heart disease or pre-existing cardiovascular disease, suggesting that the abnormal LV GLS at follow-up was likely to be related to COVID-19 infection and not a result of underlying comorbidities. Together, these findings suggest that there may be residual myocardial involvement after acute infection, regardless of disease severity. Given that the indices of longitudinal strain on STE have been shown to strongly correlate with the amount of oedema on cardiac magnetic resonance imaging and levels of lymphocytic infiltrates on endomyocardial biopsy, echocardiography with STE can serve as an essential tool in guiding the next diagnostic and therapeutic steps in patients with post-acute sequelae of COVID-19.59–62
Knowledge gaps surrounding echocardiography in coronavirus disease 2019
There are a few notable gaps in our current knowledge of echocardiographic findings in COVID-19. First, there are a limited number of studies on myocardial strain and MWE in patients with COVID-19. More STE studies will offer further information about COVID-19-related myocardial dysfunction. Additionally, studies comparing echocardiographic findings between patients with different variants of COVID-19 are lacking. Finally, there are few long-term follow-up data in the literature, and the exact clinical implications of myocardial injury and echocardiographic abnormalities during acute COVID-19 infection are still not clear. Whether myocardial injury during COVID-19 infection increases the risk of later developing cardiomyopathy, arrhythmias such as atrial fibrillation or ventricular arrhythmias, and myocardial infarction remains to be studied. Moreover, a significant limitation of existing studies is the lack of pre-infection echocardiographic data in enrolled patients, which restricts the ability to surmise any causal relationship between COVID-19 infection and echocardiography findings.
Conclusions
Echocardiography has been frequently used in acute COVID-19 infection for clinical diagnosis and management. The increasing use of STE has detected subclinical myocardial dysfunction that can be missed with standard echocardiography. A wide spectrum of echocardiographic findings, including LV and RV systolic or diastolic dysfunction, reduced basal LV strain and abnormal MWE, has been reported in patients with acute COVID-19 infection, and many of these findings correlate with biomarkers and mortality. More recently, the utility of echocardiography has expanded to guiding the diagnosis and therapeutic approaches for patients with post-acute sequelae of COVID-19. While studies including pre-infection echocardiography data are necessary to determine any true causal relationship between COVID-19 infection and the reported echocardiographic abnormalities, such studies would require leveraging established population-based cohorts that include baseline echocardiographic data regardless of cardiovascular history. Additional follow-up studies are needed to determine the long-term effects of myocardial injury during acute COVID-19 infection.