Background: Recent trial evidence suggests an early rhythm control strategy in managing atrial fibrillation (AF) reduces adverse cardiovascular outcomes. ESC guidelines recommend an integrated approach to AF management, including arrhythmia nurse specialist involvement in follow-up. Published data is limited for initiation and long-term monitoring of patients with AF on rhythm control drugs through an independent nurse-led clinic. Further to the success of our multi-disciplinary AF management pathway, in 2019, we introduced an arrhythmia nurse-led pathway for patients with AF opting for pharmacological rhythm control.1 All patients had access to a telephone helpline and had at least annual follow-up.
Aim: To evaluate short and medium-term outcomes for patients with newly diagnosed AF on pharmacological rhythm control under the independent nurse-led arrhythmia pathway. To evaluate the safety of pill in pocket (PIP) flecainide initiated without in-hospital first dose administration.
Methods: Baseline data was collected prospectively from July 2019 to December 2022 using a standardized pro forma at each visit. Outcome data including cardiovascular mortality, stroke, unplanned AF and/ or heart failure (HF) emergency department (ED) attendances and admissions was collected from the electronic patient record.
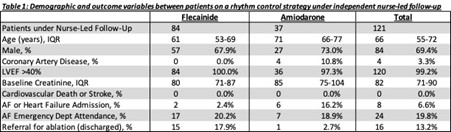
Results: Of 514 patients with AF seen in the arrhythmia service, 133 were started on medications for rhythm control – 88 on flecainide and 45 on amiodarone. Of these, 121 were under independent nurse-led followup – 84 on flecainide and 37 on amiodarone (the rest were seen in joint cardiologist-nurse clinic). Table 1 describes the cohort baseline characteristics. Twenty-two out of 84 (26.2%) on flecainide were managed with an outpatient PIP strategy, the rest were on regular flecainide (including 12 previously managed with PIP flecainide). All patients on regular flecainide had a baseline ECG and repeat ECG 1–4 weeks post-initiation. The median QRSd and QTc were as follows: QRSd pre-Fl = 96 ms versus post-Fl 99 ms, QTc pre-Fl =396 ms versus post-Fl 402 ms (no significant change). All patients on amiodarone had a baseline ECG and TFTs, and 36/37(97.3%) had baseline LFTs. All 34 patients who had PIP flecainide were initiated as OP with appropriate education and safety netting. None were hospitalized for supervised administration for first dose flecainide. Median duration of follow-up was 483 days (IQR: 267–960 days). There was no cardiovascular death or stroke (see Table 1). Twenty-four (19.8%) had an unplanned ED attendance with symptoms related to AF and/or HF, but only 8 (6.6%) were admitted to hospital. No ED attendances were related to adverse effects of flecainide or amiodarone.
Conclusion: Our initial experience shows that an independent nurseled pathway for pharmacological rhythm control in secondary care setting (without on-site facilities for catheter ablation) was both safe and effective. Medium-term follow-up of around 16 months showed zero adverse events and low rates of hospital admission due to AF. PIP flecainide initiated as OP was associated with no significant adverse events. If replicated in other similar studies, this pathway is likely to be more cost-effective without compromising patient outcomes; especially if rhythm control is more widely used in the future for AF management. ❑
Table 1: Demographic and outcome variables between patients on a rhythm control strategy under independent nurse-led follow up