Tricuspid regurgitation (TR) is a frequent finding on echocardiography, with detection rates reaching up to 86% across the different ranges of severity, with moderate or greater TR being reported in at least 6–8% of patients.1–3 The prevalence of TR increases with age, affecting >4% of people above the age of 75 years, with women being more disproportionately affected.4
The tricuspid valve (TV) apparatus has a more complex anatomy and is larger than the aortic and mitral valves. It usually consists of three leaflets: anterior, posterior and septal leaflets. The anterior leaflet is usually the largest and most mobile, and the posterior leaflet often has several scallops. The number of leaflets may vary up to seven, including accessory leaflets. The annulus is asymmetric, saddle-shaped or elliptical, with the anteroseptal aspect sitting higher towards the right atrium and the posteroseptal part sitting lowest towards the right ventricle. The annulus is dynamic according to the loading conditions, and when dilated, it becomes more flattened towards the anterior–posterior direction. Several structures come in close proximity to the TV, including the right coronary artery, coronary sinus, the atrioventricular node, the right His bundle and the non-coronary sinus of the aortic valve.5,6
There are different mechanisms for TR, which mainly include the following: (1) the primary mechanism, which is related to leaflet abnormalities, may be degenerative such as prolapse or flail leaflets, congenital as in Ebstein’s anomaly, or acquired as in endocarditis, rheumatic, carcinoid, tumours or radiation; (2) the secondary mechanism, which is the most frequent type and which can be further classified into ventricular subtype due to left-sided heart failure and/or pulmonary hypertension and is related to right ventricular enlargement, or atrial subtype related to tricuspid annular dilation; and (3) the lead-associated TR in the setting of pacemaker or defibrillator leads, which may be causative or incidental bystander in some cases.7 Distinguishing between the atrial and ventricular subtypes of secondary TR is crucial, as it may influence interventional strategies. Ventricular secondary TR is usually associated with an ellipsoid right ventricular enlargement, causing displacement of papillary muscles and leaflet tethering. On the other hand, atrial TR is associated with significant TV annular dilation and right atrial enlargement, leading to poor leaflet coaptation and a conical-shaped right ventricle.8
The diagnosis and quantification of TR may be challenging. Comprehensive assessment using 2D and 3D transthoracic echocardiography becomes crucial to quantify TR, identify its mechanism and evaluate the right ventricular size and function and the severity of pulmonary hypertension if present. Transoesophageal echocardiography is recommended for all patients considered for devices to further assess the leaflet morphology and function, number and location of TR jets and the chordae and papillary muscles. Cardiac magnetic resonance imaging may also be useful for the assessment of TR severity if there is a discrepancy in the echocardiographic findings and is the gold standard to assess the right ventricular size and function.9
TR is associated with an increased risk of mortality and morbidity. In a study of 5,223 patients, the rates of survival at 1 year were 92% with no TR, 90% with mild TR, 79% with moderate TR and 64% with severe TR.10 In another large-scale study from the Cleveland Clinic of 9,045 patients with at least moderate-to-severe TR, 44% of the patients were dead after a mean follow-up of 2.6 years.11 In the largest study to date of 439,558 adults in Australia, there was a stepwise association between worsening severity of TR and increased risk of mortality. The hazard ratios were 1.24 (1.23–1.26) with mild TR, 1.72 (1.68–1.75) with moderate TR and 2.65 (2.57–2.73) with severe TR.3 TR has also been associated with an increased risk of heart failure hospitalization and reduced functional and exercise capacity.12,13 It is important to note that prior grading systems underestimated the severity of TR, especially the extremely severe or massive and torrential grades. Hahn and Zamorano proposed an expanded grading system for TR that included categories beyond severe.14 This included a vena contracta of 7–13 mm for severe, 14–20 mm for massive and ≥21 mm for torrential TR; effective regurgitant orifice area of 40–59 mm2 for severe, 60–79 mm2 for massive and ≥80 mm2 for torrential; and 3D vena contracta area of 75–94 mm2 for severe, 95–114 mm2 for massive and ≥115 mm2 for torrential TR. These considerations become important, as these more advanced grades in the natural history of the disease carry a worse prognosis.14 There are certain parameters that may predict the progression of TR over time. In a prospective study of 1,843 patients with at least moderate TR, factors such as age, lower body mass index, chronic kidney disease, worse functional class and right ventricle dilation were associated with TR progression after 2 years of follow-up.15 Other factors, such as the presence of pacemaker and defibrillator lead, tricuspid annulus dilation and tricuspid annulus plane systolic excursion, may also predict the time to develop significant TR.16 A better understanding of these factors is important for close surveillance of these patients and intervening before reaching advanced stages of the disease.
Historically, the primary approach to treating TR has been surgical repair or replacement.17 However, these entail considerable complexity and associated risks, particularly for patients deemed high risk for conventional open-heart surgery due to several comorbidities, and an increased risk of in-hospital mortality nearing 9% with TV surgery.18,19 The operative mortality for TV surgical repair and replacement can be as high as 18 and 13%, respectively.20 This can often lead to delays in receiving treatment, resulting in further progression of disease and worse patient outcomes.21 In addition, the presence of heart failure and/or pulmonary hypertension may further complicate the management of TR.22,23 The TV has historically been considered the ‘forgotten valve’, which resulted in undertreatment. There is evidence that a longer time between the diagnosis of severe TR and surgical intervention is linked with worse long-term survival.21 Given that many patients with severe TR have multiple comorbidities, making them unsuitable for surgery, it is essential to carefully balance the risks and benefits when selecting appropriate treatment strategies.24 With the increasing evidence of worse outcomes with significant TR, it has started to gain more attention in recent years, especially with emerging transcatheter therapies offering treatment options in patients who were otherwise deemed non-surgical candidates.25 Different transcatheter tricuspid valve interventions (TTVIs) have demonstrated efficacy in addressing TR, whether primary or secondary, with lower procedural risks and shorter recovery times in comparison with open surgery.25 In this review, we will focus on the evolving landscape of TTVI for TR, explore established and emerging devices and analyse available clinical data on safety and efficacy in this rapidly growing field.
Current practices
Open-heart surgery with TV repair or replacement has been the mainstay of treating TR.17 The 2020 American College of Cardiology/American Heart Association Valvular Heart Disease guidelines gave a class I recommendation for TV surgery in patients with severe TR only at the time of left-sided valve surgery and class IIa recommendation for isolated TV surgery in patients with severe TR with associated right-sided heart failure.26 While this approach offers a definitive solution, it carries significant risks related to general anaesthesia, lengthy hospital stays and potential complications such as bleeding or infections. More importantly, a growing population of frail patients with advanced age and those with significant comorbidities are deemed inoperable.18 These limitations have fuelled the need for less-invasive alternatives. Fortunately, the landscape of TR management is undergoing a revolution with the emergence of several TTVI, which created a paradigm shift offering catheter-based approaches for these patients. Early data on these procedures are promising, with studies showing reduced complications and faster recovery times compared with open-heart surgery, particularly for high-risk patients.27,28
In an early initiative, the TriValve prospective multinational multicentre registry was developed to study outcomes in patients who underwent TTVI using multiple different devices. Procedural success was acceptable in about 75%, with associated improvement in functional status and relatively low risk of mortality.29 TTVI is still a developing field with ongoing research to determine its long-term outcomes and optimal patient selection criteria. There are three different main types of technologies.
-
Transcatheter tricuspid edge-to-edge repair (T-TEER): this approach uses a catheter to deliver a clip to grasp the native TV leaflets to promote better apposition, resulting in TR reduction. T-TEER is a more established procedure, with a growing body of evidence demonstrating its safety and efficacy for select patient populations.
-
Transcatheter tricuspid valve replacement (TTVR): this involves a catheter-based approach to deliver and implant a bioprosthetic TV. Several devices are still under investigation, with ongoing clinical trials evaluating long-term durability and effectiveness.
-
Others: heterotopic bicaval valve implantation and TV annuloplasty devices are also emerging options for the treatment of significant TR and will be discussed in further detail in this review.
Tricuspid transcatheter edge-to-edge repair
T-TEER is a minimally invasive procedure that uses a catheter-based approach to achieve better coaptation of the leaflets and minimize TR, particularly for high-risk patients who may not be suitable candidates for more invasive procedures.30 Coaptation devices, such as TriClip (Abbott Vascular, Santa Clara, CA, USA), MitraClip (Abbott Vascular) and PASCAL (Edwards Lifesciences, Irvine, CA, USA), are used to grasp the TV leaflets and facilitate better apposition by leaflet plication.31 The Dragonfly device (Venus Medtech, Hangzhou, China) is currently under investigation in China.9 TriClip is currently the only approved T-TEER device dedicated to the tricuspid position.
TriClip and MitraClip systems
Both systems use a steerable guide catheter and a clip delivery system. The components of the system consist of a steerable guide catheter, clip delivery system and a chrome-cobalt clip. In comparison with the MitraClip delivery system, the TriClip features new design changes, including two knobs in the steerable guide catheter, one knob in the steerable sleeve for tip deflection and finally a shorter radius of the distal curve. Collectively, these design changes allow better steerability of the system in the right atrium.32 Both devices are placed via 25-French transfemoral delivery systems, using the same clip. The clip is equipped with two gripper arms that are used to grasp and pull the valve leaflets and is currently available in four different implant sizes, including the fourth-generation short NT, long XT and their wide counterparts NTW and XTW, allowing tailoring to different anatomies (Figure 1A).31,33
Figure 1: Tricuspid transcatheter edge-to-edge repair systems
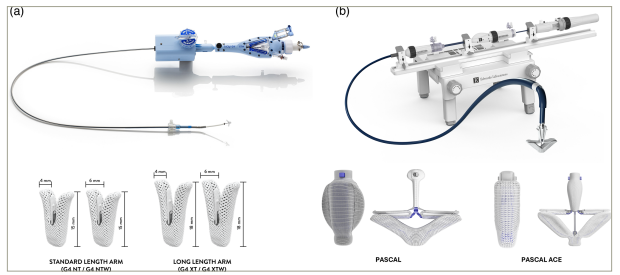
(A) TriClip (Abbott Vascular; reproduced with permission) and (B) PASCAL (Edwards Lifesciences LLC, Irvine, CA, USA; reproduced with permission) systems.
After the successful use of MitraClip in the mitral position, it was first attempted in the tricuspid position in 2015, showing promising results.34 Other studies that followed showed continued success with the MitraClip system in improving heart failure symptoms and exercise capacity in patients with severe TR. The MitraClip XT system, designed for larger coaptation gaps, demonstrated promise in reducing TR severity.35 In an analysis of 249 patients who underwent T-TEER using MitraClip in the TriValve prospective multicentre registry, procedural success was achieved in 77%. Concomitant treatment of TR and mitral regurgitation was done in 52% of the cases. At 1 year, sustained improvements in TR to moderate or less were seen in 72%. About 70% had New York Heart Association (NYHA) class I–II. Mortality was 20% and the composite of mortality or heart failure hospitalization was 35%. Procedural success was associated with better survival at 1 year in comparison with procedural failure (83 versus 69%).36 In this study, factors such as large coaptation gap ≥6.4 mm, large effective regurgitant orifice area ≥0.70 cm2, large tenting area of ≥3.2 cm2 and the absence of central or anteroseptal TR jet have been linked with higher risk of procedural failure.36
The bRIGHT study (An Observational Real-world Study Evaluating Severe Tricuspid Regurgitation Patients Treated with the Abbott TriClip™ Device; ClinicalTrials.gov identifier: NCT04483089) was a prospective, single-arm, open-label study that reported findings of TriClip use in 389 patients.37 Successful implantation was achieved in 99%, with moderate or less TR in >75% of the patients at 30 days. This was associated with improved NYHA and Kansas City Cardiomyopathy Questionary (KCCQ) at 30 days.38
The TRILUMINATE single-arm trial was designed to test the safety and efficacy of the TriClip system for the treatment of severe symptomatic TR.39 At 30 days, 86% of patients achieved a reduction in the severity of TR regurgitation by at least one grade, which was the primary endpoint for effectiveness. The primary endpoint for safety was to examine the occurrence of significant adverse events at 6 months, with a target rate of 39%. Only 6% of participants experienced major adverse events.40 After 1-year follow-up, the severity of TR decreased to a moderate grade or less in 71% of cases, as opposed to only 8% at baseline, which demonstrated repair durability. Patients in the TR group showed significant improvement in heart failure symptoms, exercise capacity and quality of life, as assessed by improvements in the NYHA functional class, 6 min walk test (6MWT) and KCCQ score at 1 year. Furthermore, there was a significant improvement in both the size and function of the right ventricle and a low rate of major adverse events and all-cause mortality.41 At 2 years, TR was moderate or lower in 60%, decreased by at least one grade in 85.4% and sustained in 75% of the participants. The improvements in heart failure symptoms, exercise capacity and quality of life remained consistent for 2 years. Nevertheless, the occurrence of all-cause mortality was documented in 18.7% of cases.42
This was followed by the TRILUMINATE Pivotal trial, which was an international, open-label, randomized controlled trial that compared the safety and effectiveness of T-TEER using the TriClip system in patients with severe symptomatic TR versus a control arm of medical therapy alone.43 Patients enrolled in the study were eligible if they had NYHA class II–IV, had pulmonary artery systolic pressure <70 mmHg, were on stable guideline-directed medical therapy for 30 days, had no other conditions requiring interventions and were deemed intermediate to high risk for surgery. The primary endpoint was a hierarchical 1-year composite that included death from any cause, TV surgery, heart failure hospitalization and improvement in quality of life defined as an increase of at least 15 points in the KCCQ. A total of 350 patients from 65 centres were enrolled with 1:1 assignment to each arm. The mean age was 78 ± 7 years, and 71% had massive or torrential TR at baseline. Successful implantation was achieved in 98.8%, with a mean of 2.2 ± 0.7 clips per patient. This was associated with a significant reduction of TR to moderate or less in 88% of the TriClip arm versus only 5.6% in the control arm at 1 year. There were associated improvements in KCCQ at 1 year by an average of 12 points in the device arm versus one point in the placebo arm. The trial met its primary endpoint with a win ratio of 1.48 and 95% confidence interval of 1.06–2.13. This was mainly driven by the improvement in quality of life; however, the incidence of death, TV surgery or heart failure hospitalization was not different between the two groups.44 The lack of improvement in survival or heart failure hospitalization could be related to the inclusion of patients with less illness in the trial compared with earlier studies. Future studies involving patients with more advanced disease and longer follow-up would be necessary to determine whether T-TEER is linked with survival benefit.45 Based on the results of this trial, the TriClip received approval from the US Food and Drug Administration (FDA) in April 2024 to become commercially available for a larger group of patients outside clinical trials.46 This marked the first time a device received panel approval based on benefits in health status and quality of life.
PASCAL system
The PASCAL device is composed of a nitinol-woven spacer positioned in the regurgitant orifice of the TV and is connected to the valve leaflets using two paddles and clasps. The device is placed through a 22-French system. There are two iterations of the device: PASCAL with 10 mm paddles and PASCAL Ace with narrower 6 mm paddles (Figure 1B). The device has a unique elongation feature, which may reduce the risk of entanglement with the subvalvular apparatus.47
In an observational study, the compassionate use of PASCAL in patients with challenging TV anatomy and severe TR showed high procedural success in 86% of participants at 30 days.48 Furthermore, a notable clinical improvement was observed, including two-grade reduction in TR severity in 85% of the patients, a decrease in heart failure symptoms and improvement in exercise capacity.48 The PASTE (PASCAL for Tricuspid Regurgitation – A European Registry; ClinicalTrials.gov identifier: NCT05328284) registry retrospectively reported findings for the commercial use of PASCAL and PASCAL Ace in 235 patients.48,49 The procedural success was 78%, and 78% had moderate or less TR at the latest follow-up. Similar improvements in NYHA were noted.50 The TriCLASP (Transcatheter Repair of Tricuspid Regurgitation with Edwards PASCAL Transcatheter Valve Repair System: A European Prospective, Multicenter Post-market Clinical Follow up; ClinicalTrials.gov identifier: NCT04614402) study was a prospective, single-arm study that evaluated the safety and performance of the PASCAL system.51 This study demonstrated a significant and sustained reduction in TR grade to moderate or less in 90% of patients at 30 days. Major adverse events occurred in 3%, and significant improvements in heart failure symptoms, exercise capacity and quality of life were observed.52 Another small, observational, propensity-matched analysis compared PASCAL and MitraClip XT for the treatment of severe symptomatic TR in 44 patients and noted similar efficacy and safety with comparable short-term outcomes.53
The CLASP-TR (Edwards PASCAL TrAnScatheter Valve RePair System in Tricuspid Regurgitation Early Feasibility Study; ClinicalTrials.gov identifier: NCT03745313) single-arm, early feasibility study analysed the outcomes of PASCAL use in 65 patients with severe TR who remained symptomatic despite medical therapy.54 At 1 year, 100% of patients had at least one-grade reduction in TR, including 86% with moderate or less residual TR. There were significant improvements in NYHA, KCCQ and 6MWT. About 12% died and 21.5% were hospitalized for heart failure by 1 year.55 The CLASP-II TR Pivotal trial (A Prospective, Multicenter, Randomized, Controlled Pivotal Trial to Evaluate the Safety and Effectiveness of Transcatheter Tricuspid Valve Repair with the Edwards PASCAL Transcatheter Valve Repair System and Optimal Medical Therapy [OMT] Compared to OMT Alone in Patients with Tricuspid Regurgitation; ClinicalTrials.gov identifier: NCT04097145) is a prospective, multicentre, randomized controlled trial that is currently undergoing to compare the safety and efficacy of the PASCAL system in patients with severe TR in comparison with medical therapy alone (Table 1).35,36,38,40–42,44,48,50,52,53,55,56
Table 1: Tricuspid transcatheter edge-to-edge repair systems35,36,38,40–42,44,48,50,52,53,55
| Device | Study name | Number of patients | Follow-up time | Moderate or less TR | NYHA class I-II | Change in KCCQ | Change in 6MWT | All-cause mortality (%) | CV mortality (%) | HFH (%) |
| MitraClip (Abbott Vascular) | Ruf et al.35 | 50 | 30 days | 14% → 54% | 2% → 56% | – | +68 m | 0 | 0 | 0 |
| TriValve registry36 | 249 | 1 year | 3.2% → 77.1% | 4.4% → 69.1% | – | – | 19 | – | – | |
| TriClip (Abbott Vascular) | bRIGHT registry38 | 511 | 30 days | 2% → 77% | 20% → 79% | +19 points | – | 1 | 0.8 | – |
| TRILUMINATE single-arm study40 | 85 | 6 months | 6% → 57% | 25% → 86% | +18.4 points | +54.6 m | 5 | 4 | – | |
| TRILUMINATE single-arm study41 | 85 | 1 year | 8% → 71% | 31% → 83% | +20 points | +31 m | 7.1 | 4.8 | -40 | |
| TRILUMINATE single-arm study42 | 85 | 2 years | 4% → 60% | 33% → 81% | +13 points | +61 m | 16.7 | 13.1 | -84 | |
| TRILUMINATE pivotal randomized controlled trial44 | 360 (TriClip: 175; control: 175) | 1 year | TriClip: 3% → 87% versus control: 2% → 4.8% | TriClip: 45.6% → 83.9% versus control: 46.6% → 59.5% | TriClip: +12.3 points versus control: +0.6 points | TriClip: -8.1 m versus control: -25.2 m | TriClip: 8.8 versus control: 7.7 | TriClip: 6.5 versus control: 4.7 | TriClip: 14.9 versus control: 12.1 | |
| PASCAL (Edwards Lifesciences) | Fam et al.48 | 28 | 30 days | 0% → 85% | 0% → 88% | – | +95 m | 7.1 | – | 3.5 |
| PASTE registry50 | 235 | 6 months | 9% → 78% | 10% → 67% | – | +39 m | 12 | 6 | 13 | |
| TriCLASP study52 | 74 | 3 months | 0% → 90% | 23.1% → 55.7% | +13.4 points | +38.2 m | 2.9 | 1.5 | 4.5 | |
| Sugiura et al.53 | 44 (PASCAL: 22; MitraClip: 22) | 30 days | PASCAL: 0% → 50% and MitraClip: 0% → 68% | PASCAL: 5% → 93% and MitraClip: 9% → 82% | – | +32.9 m | PASCAL: 5 and MitraClip: 5 | – | – | |
| CLASP-TR study55 | 65 | 1 year | 3.1% → 86% | 29.2% → 92% | +18 points | +94 m | 10.8 | 7.7 | 18.5 |
CV = cardiovascular; HFH = heart failure hospitalization; KCCQ = Kansas City Cardiomyopathy Questionnaire; 6MWT = 6 minute walk test; NYHA = New York Heart Association; TR = tricuspid regurgitation.
Transcatheter tricuspid valve replacement
Several TTVR devices have emerged to offer alternative options for high-risk patients. Early results from clinical studies are promising, demonstrating significant improvements in functional status, quality of life and right ventricular remodelling in patients with severe TR.22,25 A key advantage of TTVR is its potential to eliminate TR. In addition, the variety of TTVR devices available allows for treating a wider range of anatomical variations and pathologies.57
While TTVR offers promising benefits, it is important to acknowledge its potential complications. These may include atrioventricular block, interaction with or damage to existing pacemaker/defibrillator leads, difficulties implanting pacemakers in the future and risk of worsening right ventricular function.25
Unlike surgical valve replacements, which rely less on pre-approval trials, TTVR requires rigorous large-scale randomized controlled trials, which can be costly and time-consuming.58 Despite these challenges, ongoing research and technological advancements are rapidly shaping the field of TTVR, continuously improving the feasibility and outcomes of these procedures. We will explore the different types of TTVR devices and discuss recent studies in more detail as follows.
Evoque system
The Evoque valve (Edwards Lifesciences) is a self-expanding nitinol frame valve with bovine pericardial leaflets and sealing skirt and nine anchors. It is available in three different sizes, including 44, 48 and 52 mm, and is delivered through a 28-French transfemoral approach (Figure 2A).59
Figure 2: Transcatheter tricuspid valve replacement systems
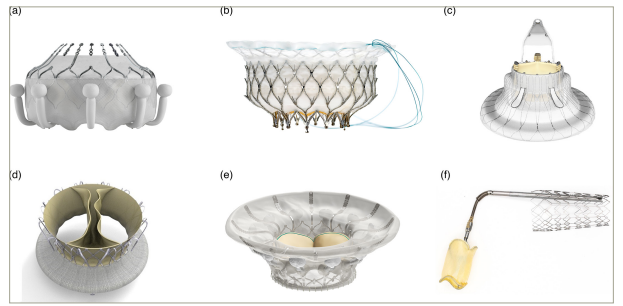
(A) Evoque valve (Edwards Lifesciences, Irvine, CA, USA; reproduced with permission), (B) Intrepid valve (Medtronic, Minneapolis, MN, USA; the Intrepid™ transcatheter mitral valve replacement system is an investigational device; limited by federal [US] law to investigational use; exclusively for clinical investigations; not approved by the Food and Drug Administration and not for sale in the USA), (C) LuX-Valve (Jenscare Biotechnology, Ningbo, China), (D) Trisol valve (Trisol Medical, Yokneam, Israel), (E) CardioValve (Venus Medtech, Hangzhou, China) and (F) CroíValve system (CroíValve, Dublin, Ireland). All images were reproduced with the permission of the copyright holders.
In a preliminary, observational, non-randomized, single-arm study, 25 patients with severe symptomatic TR were enrolled in a compassionate use of the Evoque valve system at six different centres. Inclusion criteria included right-sided heart failure with NYHA functional class II–IV despite medical therapy and being deemed at high surgical risk or inoperable by the local heart team. The technical success was 92%, with no conversions to surgery or procedural deaths. At 30 days, 96% had moderate or less TR, and 76% had NYHA class I–II, with a 0% mortality rate.60 At 1-year follow-up, 96% still had moderate or less TR, and only two patients died.61
In the TRISCEND study of 56 patients with moderate or greater symptomatic TR, TTVR using the Evoque valve was associated with a reduction of TR to mild or less in 98%.62 Major adverse events at 30 days included 1 death due to failed procedures, 2 device embolization resulting in reinterventions, 15 severe bleeding and 1 major access-site complication. There were also improvements in functional status in 80%.63 In an analysis of 1-year outcomes of 176 patients from the TRISCEND study, TR continued to be mild or less in 98%, with improvements in stroke volume and cardiac output. About 93% of patients had NYHA class I–II, with increases in KCCQ score and 6MWT distances. Mortality occurred in 9% and heart failure hospitalization in 10% at 1 year.64
The TRISCEND II Pivotal randomized controlled trial (Edwards EVOQUE Transcatheter Tricuspid Valve Replacement: Pivotal Clinical Investigation of Safety and Clinical Efficacy Using a Novel Device; ClinicalTrials.gov identifier: NCT04482062) is an open-label trial that evaluated the safety and effectiveness of the Evoque TTVR system plus optimal medical therapy versus medical therapy alone in 400 patients.65 The 30-day primary endpoint for safety is a composite of major adverse events, whereas the 6-month primary endpoint for effectiveness involves a reduction in TR severity and a hierarchical composite of KCCQ (improvement ≥10 points), NYHA (improvement ≥1 class) and 6MWT (improvement ≥30 m). Finally, the 1-year primary endpoint involves a hierarchical composite of mortality, implantation of right ventricular assist device of heart transplant, TV surgery or intervention, annualized heart failure hospitalization and KCCQ/NYHA/6MWT. Preliminary results were presented in late 2023 for the first 150 patients, who completed 6-month follow-up, including 96 who received Evoque TTVR demonstrating the safety and effectiveness of the system.66 Major adverse events occurred in 27.4%, which is less than the expected rate of 43.8%. About 94% had mild or less TR, including 78% with trace or less TR. There were also associated improvements in functional status with a win ratio of 4.6. Findings of the total cohort will be presented later in 2024, including the 1-year hierarchical composite.66 The 1-year results of the TRISCEND-II trial were recently published, showing superiority of TTVR using the EVOQUE system for treatment of severe TR compared with medical therapy.67 The trial met its primary endpoint with a win ratio favouring TTVR (2.02; 95% confidence interval 1.56–2.62). This was mainly driven by improved symptoms and quality of life. However, there were no significant differences in mortality or heart failure hospitalizations compared with medical therapy. Notably, there were higher rates of severe bleeding and new pacemaker requirement in the EVOQUE arm. The Evoque valve received regulatory FDA approval for the treatment of severe symptomatic TR in February 2024 to become the first dedicated TTVR system to reach that milestone and be commercially available to a larger group of patients outside clinical trials.68
NaviGate system
The first TTVR with a dedicated system was performed using the NaviGate valve (NaviGate Cardiac Structures, Inc., Lake Forest, CA, USA). It is a tri-leaflet bioprosthesis made from equine pericardium mounted on a self-expanding nitinol stent. It is available in five different sizes, including 36, 40, 44, 48 and 52 mm, and is placed through a direct transatrial access using a 45-French sheath.69
In a case series of five patients with severe TR who underwent compassionate implantation of the NaviGate valve, successful TTVR was achieved in all five with no need for cardiopulmonary bypass or ventricular pacing. There was one in-hospital death with a complicated post-operative course requiring dialysis and mechanical ventilation. Three patients of the five had significant bleeding and one patient required re-exploration. At 3–6 months, there were improvements in symptoms, right ventricular remodelling and an increase in cardiac output.69 The transatrial approach has limited the widespread adoption of this system.
Intrepid system
The Intrepid device (Medtronic, Minneapolis, MN, USA) can be used for mitral or tricuspid valve replacement. It was originally designed for transapical implantation but can be done via a transfemoral approach using 35-French delivery system.70 The Intrepid device features a dual-structure design. There is an inner circular stent that houses a tri-leaflet bovine pericardial valve, in addition to a conformable outer fixation stent with a flexible atrial brim that provides additional anchoring. The outer frame has three different sizes, including 43, 46 and 50 mm, while the inner stent frame is 27 mm in diameter (Figure 2B). One potential advantage is the ability to recapture and retrieve the valve during the implantation process.70,71 The APOLLO clinical trial (Transcatheter Mitral Valve Replacement with the Medtronic Intrepid TMVR System in Patients with Severe Symptomatic Mitral Regurgitation; ClinicalTrials.gov identifier: NCT03242642) is currently enrolling for Intrepid valve implantation in patients with severe mitral regurgitation. An early feasibility, multicentre, prospective, non-randomized study of the Intrepid valve in the tricuspid position is currently recruiting (The Early Feasibility Study of the Transcatheter Tricuspid Valve Replacement System Transfemoral System; ClinicalTrials.gov identifier: NCT04433065).
LuX-Valve system
The LuX-Valve (Jenscare Biotechnology, Ningbo, China) is a tri-leaflet bioprosthetic heart valve made from bovine pericardium mounted on a self-expanding nitinol stent. It includes an atrial disc and an interventricular septal anchor with expanded polytetrafluoroethylene-covered graspers for secure placement (Figure 2C). The first generation of the LuX-Valve is delivered through a right thoracotomy and transatrial approach using a 32-French catheter. Guidance during implantation is achieved with transoesophageal echocardiography and fluoroscopy. Early experience in China included implantation in 12 patients with promising results.72 The LuX-Valve Plus is the second generation that is implanted through a transjugular approach. There are no available data regarding its outcomes, which are currently limited to compassionate use.70
Trisol valve system
The Trisol valve (Trisol Medical, Yokneam, Israel) comprises a single leaflet, and the leaflet is affixed by two commissures, enabling it to function as a bi-leaflet valve (Figure 2D). The device design allows slower closing of the leaflets, which is intended to preserve the right ventricular function following the valve replacement and reduce the risk of conduction problems. An early feasibility study is currently underway after the successful implantation of two cases in the USA with satisfactory results.73
CardioValve system
The CardioValve (Venus Medtech, Yehuda, Israel) TTVR system is a novel technology currently undergoing clinical evaluation. This system uses a bioprosthetic valve composed of three bovine pericardial leaflets and a dual self-expanding nitinol frame. The system is delivered via a 32-French transfemoral approach. The CardioValve boasts 24 grasping points designed for secure and atraumatic anchoring to the native valve annulus (Figure 2E). The initial clinical experience is limited to case reports, highlighting the early stage of development for this promising TTVR technology.74
CroíValve system
The Duo CroíValve system (CroíValve, Dublin, Ireland) features a unique design combining a coaptation spacer valve with a superior vena cava stent anchor system (Figure 2F). This design aims to overcome anatomical limitations encountered with traditional approaches, potentially benefiting a broader range of patients. In addition, the CroíValve system uses a single-size valve and delivery system, simplifying the procedure. Notably, it leverages the internal jugular vein as the access point for deployment, offering a less-invasive alternative to other techniques, potentially leading to faster recovery times and less patient discomfort.30
The first successful human implants of the Duo CroíValve for the treatment of TR were done as part of the TANDEM I clinical trial study (A European Feasibility Study of the CroíValve DUO Transcatheter Tricuspid Coaptation Valve System in Patients With Tricuspid Regurgitation; ClinicalTrials.gov identifier: NCT05296148) in Poland in 2023 (DUO Coaptation Valve [CroíValve] system for treating tricuspid regurgitation).75,76 This initial experience demonstrated the feasibility and potential of the CroíValve system. However, further large-scale clinical trials are necessary to establish its long-term safety and efficacy in treating TR. Ongoing clinical investigations will play a crucial role in determining the utility of the CroíValve system in the landscape of TR management (Table 2).60,61,63,64,66,67,69,72
Table 2: Transcatheter tricuspid valve replacement systems60,61,63,64,66,67,69,72
| Device | Study name | Number of patients | Follow-up time | Moderate or less TR | NYHA class I–II | Change in KCCQ | Change in 6MWT | All-cause mortality (%) | CV mortality (%) | Hfh (%) |
| Evoque (Edwards Lifesciences) | Fam et al.60 | 25 | 30 days | 0% → 96% | 12% → 76% | – | – | 0 | 0 | 0 |
| Webb et al.61 | 27 | 1 year | 0% → 96% | 11% → 69% | – | +67 m | 7 | 0 | 7 | |
| TRISCEND single-arm study63 | 56 | 30 days | 9.6% → 100% | 12.5% → 78.8% | +19 points | +49.8 m | 3.6 | 1.8 | – | |
| TRISCEND single-arm study64 | 176 | 1 year | 12.5% → 100% | 25.2% → 93.3% | +25.7 points | +56.2 m | 9.1 | – | 10.2 | |
| TRISCEND II randomized controlled trial preliminary analysis66,67 | 400 (Evoque: 267 and control: 133) | 1 year | Evoque: 0% → 99.1% versus control: 0% → 16.1% | Evoque: 22.6% → 90.0% versus control: 29.3% → 34.2% | Evoque: +21.5 points versus control: +3.7 points (6 months) | Evoque: +10.6 meters versus control: −20.3 m (6 months) | Evoque: 11.6 versus control: 10.5 | Evoque: 8.5 versus control: 7.5 | Evoque: 20.9 versus control: 26.1 | |
| Navigate (NaviGate) | Elgharably et al.69 | 5 | 6 months | 0% → 100% | – | – | – | 20 | 0 | 0 |
| LuX-Valve (Jenscare Biotechnology) | Lu et al.72 | 17 | 30 days | 0% → 100% | 0% → 54.5% | – | +99.8 m | 8.3 | 8.3 | – |
CV = cardiovascular; HFH = heart failure hospitalization; KCCQ = Kansas City Cardiomyopathy Questionary; 6MWT = 6 minute walk test; NYHA = New York Heart Association; TR = tricuspid regurgitation.
Transcatheter heterotopic caval valve implantation
In contrast to orthotopic valve implantation in the TV position, heterotopic implantation of valves in the superior and inferior venae cavae offers an alternative approach to the treatment of severe TR in patients with unfavourable TV anatomy. Caval valve implantation (CAVI) reduces the congestive effect of severe TR. Important considerations for this option involve a careful assessment of the caval anatomy due to the risk of embolization, as well as valve thrombosis with the relatively slower flow.70 This approach was first described in 2011 using self-expanding custom-made valves that were implanted in the inferior vena cava.77 The SAPIEN-XT valve (Edwards Lifesciences) was also previously attempted in the bicaval positions back in 2013.78 The TRICAVAL study (Treatment of Severe Secondary TRIcuspid Regurgitation in Patients with Advance Heart Failure with CAval Vein Implantation of the Edwards Sapien XT VALve; ClinicalTrials.gov identifier: NCT02387697) compared outcomes with bicaval SAPIEN-XT versus medical therapy in 28 patients, which showed no differences in outcomes, but the trial was prematurely stopped due to safety concerns regarding valve embolization.79,80 Several dedicated systems have since emerged for heterotopic CAVI as mentioned below.
TricValve system
TricValve (P&F Products and Features, Vienna, Austria) has emerged as a leading contender for the management of patients with severe TR. This involves the placement of two self-expandable valves in the superior (25 or 29 mm) and inferior venae cavae (31 or 35 mm) via a 24-French delivery system (Figure 3A). The procedure is relatively straightforward compared with orthotopic TTVR. An important consideration is the presence of significant caval backflow to warrant the benefit from the TricValve; thus, the presence of V-wave of 25 mmHg has been proposed as one of the criteria for eligibility.70
Figure 3: Transcatheter heterotopic caval valve implantation systems
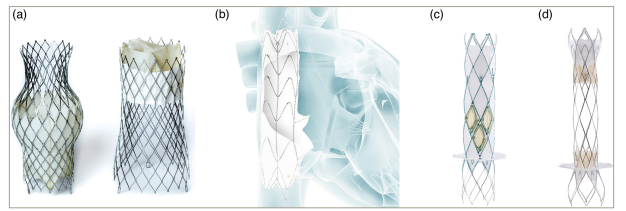
(A) TricValve system (P&F Products and Features; reproduced with permission), (B) TriCento system (New Valve Technology, Hechingen, Germany), (C) Trillium system (Innoventric, Ness Ziona, Israel) and (D) Unica system (Innoventric). All images were reproduced with the permission of the copyright holders.
The TRICUS EURO study (Safety and Efficacy of the TricValve® Transcatheter Bicaval Valves System in the Superior and Inferior Vena Cava in Patients with Severe Tricuspid Regurgitation; ClinicalTrials.gov identifier: NCT04141137) included 35 patients who underwent TricValve implantation.81 The procedural success was 94%, with no procedural mortality or conversion to open surgery. At 6 months, there were significant improvements in health status, with 8.5% mortality and 20% hospitalization for heart failure.82 One-year follow-up of 44 patients from the TRICUS (Safety and Efficacy of the TricValve® Transcatheter Bicaval Valves System in the Superior and Inferior Vena Cava in Patients with Severe Tricuspid Regurgitation; ClinicalTrials.gov identifier: NCT03723239) and TRICUS EURO studies showed persistent clinical improvement with low mortality rates.83 There were no documented stent fractures, valve thrombosis or conduction abnormalities.84 The TRICAV trial (A Prospective, Multicenter Clinical Trial of the TricValve® Transcatheter Bicaval Valve System in Subjects with Severe Tricuspid Regurgitation; ClinicalTrials.gov identifier: NCT06137807) is a prospective, multicentre, single-arm trial that is currently enrolling in the USA.85
TriCento system
TriCento (New Valve Technology, Hechingen, Germany) features a nitinol-covered stent with porcine pericardial leaflets serving as a valve to prevent the systolic backflow in the venae cavae (Figure 3B). It also involves radio-opaque markers allowing accurate bicaval positioning. The device is implanted via a transfemoral approach with a 24-French sheath.86
In a report of 21 patients who underwent CAVI using the TriCento device, technical success was achieved in all patients, with no procedural or in-hospital deaths. About 65% of patients had NYHA class I–II, and the 1-year survival rate was 76%. Evidence of asymptomatic fracture was noted in three patients by computed tomography without affecting the device function.87
Trillium and Unica systems
The Trillium device (Innoventric, Ness Ziona, Israel) involves a bare metal stent with struts and a sealing skirt in one connected design. The stent is placed at the level of the superior vena cava, whereas the sealing skirt is positioned in the inferior vena cava (Figure 3C).88 An early feasibility study for this device is currently underway (Prospective, Single Arm, Multi-center, First-in-human [FIH] Study to Assess the Safety and Performance of the Innoventric Trillium™ Stent Graft for the Treatment of Severe Tricuspid Regurgitation [TR]; ClinicalTrials.gov identifier: NCT04289870).89 Another iteration of heterotopic CAVI by the same company is the Unica cross-valve (Innoventric), which involves two intra-luminal valves that are placed in the superior and inferior vena cavae, and a bare metal centre structure in-between that allows future crossing to perform TTVR or other transseptal procedures (Figure 3D and Table 3).82,84,87,90
Table 3: Transcatheter heterotopic caval valve implantation systems82,84,87
| Device | Study name | Number of patients | Follow-up time | Moderate or less TR | NYHA class I–II | Change in KCCQ | Change in 6MWT | All-cause mortality (%) | CV mortality (%) | HFH (%) |
| TricValve (P&F Products) | TRICUS EURO Study82 | 35 | 6 months | 0% → 13.6% | 0% → 79.4% | +17.7 points | +31 m | 8.5 | 0 | 20 |
| TRICUS and TRICUS EURO84 | 44 | 1 year | 0% → 0% | 0% → 62.2% | +15 points | +41 m | 6.8 | 2.2 | 29.5 | |
| TriCento (New Valve Technology) | Wild et al.87 | 21 | 1 year | 0% → 12% | 4.8% → 65% | – | – | 24 | 10 | 19 |
CV = cardiovascular; HFH = heart failure hospitalization; KCCQ = Kansas City Cardiomyopathy Questionary; 6MWT = 6 minute walk test; NYHA = New York Heart Association; TR = tricuspid regurgitation.
Transcatheter tricuspid annuloplasty devices
The concept of annuloplasty was initially introduced in the surgical repair of the mitral and tricuspid valves. There are several transcatheter technologies that emerged as potential options, which may prove useful, especially in patients with severe functional TR.33
Cardioband system
The Cardioband system (Edwards Lifesciences) represents the most used TV annuloplasty device in patients with severe functional TR. Cardioband consists of a flexible ring that is positioned around the atrial aspect of the TV annulus via a 26-French transfemoral access. The device is cinched after securing in place to effectively reduce the annular diameter (Figure 4A).91
Figure 4: Transcatheter tricuspid annuloplasty devices
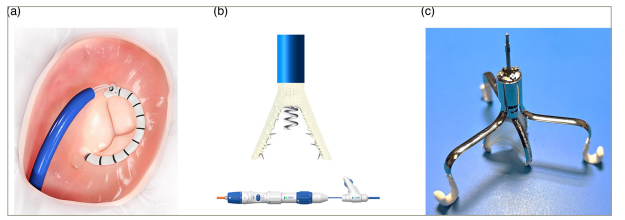
(A) Cardioband (Edwards Lifesciences, Irvine, CA, USA), (B) K-Clip (Huihe, Shanghai, China) and (C) Tricuspid flow optimizer, also known as TRiFlO device (TriFlo Cardiovascular, Newport Beach, CA, USA). All images were reproduced with the permission of the copyright holders.
TriBAND (Thirty-day outcomes of the Cardioband tricuspid system for patients with symptomatic functional tricuspid regurgitation) was a single-arm, multicentre, prospective study in Europe that evaluated the short-term outcomes of Cardioband in 61 patients with severe functional TR.92 Device success was achieved in 97%, with improvements in TR to moderate or less in 78%. At 30 days, mortality was 1.6% and the reduction in TR was durable with associated improvement in health status.92 In the prospective multicentre TRI-REPAIR (TrIcuspid Regurgitation RePAIr with CaRdioband Transcatheter System; ClinicalTrials.gov identifier: NCT02981953) study, 2-year outcomes of the Cardioband system were reported.93 Technical success was achieved in all 30 patients, with 16% reduction in the septolateral annular diameter, and 72% of the patients had moderate or less TR. Notably, 82% of patients had NYHA class I–II at 2 years with improved 6MWT and KCCQ scores. Another prospective study evaluated the 1-year outcomes of the device, which demonstrated positive sustained results. The rate of death from any cause, hospitalizations for heart failure and repeat procedures were all low, supporting the device’s safety record established in European studies.94
With promising results, there are still some limitations regarding the Cardioband system, including that it may not be suitable for all TR patients with severe anatomical leaflet issues or excessive right ventricle enlargement, and long-term data on its effectiveness and durability of repair are still limited. While it focuses on reducing the size of the valve opening, it may not address other underlying causes of TR, such as degenerative leaflets.91
TriCinch system
The TriCinch device (4Tech Cardio, Galway, Ireland) was designed for the reduction of the annular size to improve TR. It consists of a stainless steel corkscrew implant and a self-expanding nitinol stent that are connected together by a band. The corkscrew implant is anchored to the anterior aspect of the TV annulus and the stent is deployed in the inferior vena cava below the hepatic region. The band then pulls the anchor to plicate the annulus. The device is placed via a transfemoral approach using a 24-French sheath.95
Early data in 24 patients, presented in 2017, showed procedural success in 75%. However, at 1 year, the rates of anchor detachment and the haemopericardium were relatively high. A newer generation of the device was being developed to address these concerns, but the trial was later terminated and the device is not currently available.96
TriAlign system
The Mitralign device (Mitralign, Tewksbury, MA, USA) was originally developed for the treatment of functional mitral regurgitation in 2013, which showed promising results.97 The device was adapted in 2015 to facilitate TV annuloplasty as a first-in-human experience.98 It involves the sequential delivery of polyester pledgets at the anteroposterior and septal posterior commissures, through a transjugular approach. The pledgets are then cinched together, thus reducing the TV annular size.99
In the prospective, single-arm, multicentre, early feasibility SCOUT study (Early Feasibility of the Mitralign Percutaneous Tricuspid Valve Annuloplasty System [PTVAS] for Symptomatic Chronic Functional Tricuspid Regurgitation; ClinicalTrials.gov identifier: NCT02574650), the use of TriAlign in 15 patients was technically successful in 80% at 30 days.100 This resulted in reductions in the tricuspid annulus and effective orifice area. These were associated with improvements in functional status.101 Results from the SCOUT-II trial (Safety and Performance of the Trialign Percutaneous Tricuspid Valve Annuloplasty System (PTVAS) for Symptomatic Chronic Functional Tricuspid Regurgitation; ClinicalTrials.gov identifier: NCT03225612) were presented in 2018, showing device success in 82% with similar reductions in annulus and improvements in health status.102 Despite that, the device was abandoned and is no longer under development.9
K-Clip system
The K-Clip (Huihe, Shanghai, China) is a newer technology that consists of a 4 mm screw-shaped anchor and a clip with two arms. The first step is to screw the anchor to the posterior aspect of the annulus, which is then retracted to pull the tissue between the two arms of the clip, hence reducing the orifice area (Figure 4B). It is available in multiple sizes (12, 14, 16 and 18 mm) and is placed via a transjugular approach through an 18-French delivery system.70
In preliminary data collected from a prospective, multicentre, single-arm study, published in 2023, the compassionate use of K-Clip in 15 patients with severe symptomatic TR resulted in reductions of the TV circumference by 14% and area by 25%, in addition to improved TR grade by at least two grades in 60%. There were improvements in functional status noted by the NYHA class and KCCQ. The rates of complications were low (Table 4).92–94,101–103
Table 4: Transcatheter tricuspid annuloplasty devices92–94,101–103
| Device | Study name | Number of patients | Follow-up time | Moderate or less TR | NYHA class I–II | Change in KCCQ | Change in 6MWT | All-cause mortality (%) | CV mortality (%) | HFH (%) |
| Cardioband (Edwards Lifesciences) | TriBAND study92 | 61 | 30 days | 6% → 69% | 15% → 74% | +17 points | – | 1.6 | 0 | – |
| TRI-REPAIR study93 | 30 | 2 years | 24% → 72% | 17% → 82% | +14 points | +73 m | 26.7 | 0 | 44 | |
| Gray et al.94 | 37 | 1 year | 0% → 73% | 35.1% → 92.3% | +19 points | +7.2 m | 13.5 | 8.1 | 10.8 | |
| TriAlign (Mitralign) | SCOUT study101 | 15 | 30 days | – | 33.3% → 100% | – | +52.9 m | 0 | 0 | – |
| SCOUT-II study preliminary analysis102 | 39 | 30 days | – | 30.3% → 97% | – | +44 m | 0 | 0 | – | |
| K-Clip (Huihe) | Zhang et al.103 | 15 | 30 days | 0% → 66.7% | 6.7% → 86.7% | +15.6 points | +39.54 m | 0 | 0 | 0 |
CV = cardiovascular; HFH = heart failure hospitalization; KCCQ = Kansas City Cardiomyopathy Questionary; 6MWT = 6 minute walk test; NYHA = New York Heart Association; TR = tricuspid regurgitation.
Other technologies
FORMA spacer system
The FORMA Repair System (Edwards Lifesciences) was designed to facilitate the coaptation of the TV leaflets by occupying the TV orifice as a spacer device. It consists of a balloon spacer and a rail. The rail is advanced and anchored at the right ventricular apex. Once in position, a 12 mm or 15 mm spacer is expanded to reduce the regurgitant orifice area and allow better coaptation of the leaflets onto the spacer. The procedure requires a surgical cutdown through the subclavian or axillary vein.104
A prospective, multicentre trial was conducted to assess the feasibility and effectiveness of the FORMA Repair System in patients with symptomatic TR. It demonstrated a high rate of procedural success, with a significant reduction in TR severity. Patients experienced significant improvements in symptoms and functional status following the procedure. The FORMA Repair System demonstrated a favourable safety profile, with low rates of procedural complications and adverse events. Major adverse events were infrequent and mostly manageable.104 Long-term follow-up data indicated sustained improvements in TR severity and clinical outcomes, suggesting the durability of the FORMA Repair over time.105 Despite promising initial results, the production of the FORMA system is currently halted.
Tricuspid flow optimizer
The TRiFlO device (TriFlo Cardiovascular, Newport Beach, CA, USA) has a unique design consisting of three anchors that attach to the commissures of the native TV annulus in an atraumatic fashion. The device then opens during systole and closes during diastole, targeting the TR jet with minimal interaction with the right ventricle (Figure 4C). It is placed via a 37-French sheath and is fully repositionable and recapturable.106
First-in-human experience showed a reduction in TR from torrential to moderate, which remained durable at 6 months with reverse remodelling of the right ventricle.106 Early feasibility study is currently enrolling (Transcatheter Treatment of Tricuspid Valve Regurgitation with the TriFlo Tricuspid Flow Optimizer [TFO] System; ClinicalTrials.gov identifier: NCT06093828).107
Mistral system
The Mistral device (Mitralix, Tel Aviv, Israel) was designed for transcatheter chordal repair. It can be implanted either in the left ventricle in cases of functional mitral regurgitation or in the right ventricle for the management of functional TR. It involves a spiral-shaped nitinol wire that facilitates the grasping of the chordae tendineae to bring it closer to the leaflets and create a ‘flower bouquet’ shape. The device is delivered via an 8.5-French steerable guide catheter and a 7.5-French Mistral delivery system.108
An early feasibility study in seven patients showed procedural success in 100%, with a reduction of TR by at least one grade in all patients. There were reductions in effective regurgitant orifice area and regurgitant volume and associated improvements in 6MWT, KCCQ and NYHA. No adverse events were reported at 30 days.108 The TRIBUTE Pivotal study (Mistral Percutaneous Tricuspid Valve Durable Repair Pivotal Study; ClinicalTrials.gov identifier: NCT05767645) is an open-label, single-arm, prospective trial that is currently enrolling to evaluate the efficacy at
30 days and safety at 6 months (Table 5).104,105,108,109
Table 5: Other transcatheter valve tricuspid interventions104,105,108
| Device | Study name | Number of patients | Follow-up time | Moderate or less TR | NYHA class I–II | Change in KCCQ | Change in 6MWT | All-cause mortality (%) | CV mortality (%) | HFH (%) |
| FORMA Spacer (Edwards Lifesciences) | Puri et al.104 | 7 | 30 days | 0 % → 100% | – | +26.6 points | +29 m | 0 | 0 | – |
| Asmarats et al.105 | 19 | 2–3 years | 0 % → 33% | 5.3 % → 67% | +16.2 points | +54 m | 23.5 | 17.6 | 17.6 | |
| Mistral (Mitralix) | MATTERS Study108 | 7 | 30 days | Improved by at least one grade in 100% | 0 % → 100% | +20.2 points | +102 m | 0 | 0 | 0 |
CV = cardiovascular; HFH = heart failure hospitalization; KCCQ = Kansas City Cardiomyopathy Questionary; 6MWT = 6 minute walk test; NYHA = New York Heart Association; TR = tricuspid regurgitation.
Decision-making and device selection
Careful patient selection is crucial for successful TTVI, as it improves patient outcomes and helps avoid potential harm from devices that may not benefit certain groups of patients. There are limited data on how to select the appropriate devices for different patients, especially in the absence of direct comparisons across different devices. Multimodality imaging plays a significant role in planning for TTVI both before and during the procedure. Cardiac computed tomography can be used to identify the anatomy of the TV apparatus, assess device eligibility and plan for device delivery. Intraprocedural imaging relies mainly on transoesophageal echocardiography and fluoroscopy. Fusion imaging may also assist in device delivery to ensure accurate positioning. Furthermore, the use of 3D intracardiac echocardiography can be very helpful, especially when the image quality by transoesophageal echocardiography is not adequate.9
After failed medical management of TR, heart team discussions can help determine candidacy for surgical versus transcatheter options. In a review article, Maisano et al. highlighted some of the technical and anatomical limitations related to TTVI, which may preclude those therapies for certain groups of patients.9 First, the success of T-TEER depends on several factors, such as coaptation gap, number of leaflets and intraprocedural imaging quality. The presence of device leads may make the procedure more challenging, but it can still be considered. Second, TTVR may be limited by the size and shape of the TV annulus and subvalvular apparatus, as well as the size of the right ventricle and veins. Finally, TV annuloplasty may be constrained by the degree of right ventricular remodelling, the distance from the right coronary artery and other technical challenges related to the device implantation and imaging.9
The authors then proposed different algorithms according to the mechanism of TR. In patients with functional TR, a detailed stepwise approach is needed, which initially involves the differentiation between atrial and ventricular functional TR via transoesophageal echocardiogram assessment. For the ventricular subtype, right heart catheterization is necessary to determine right ventricular function and pulmonary pressures. In cases of poor right ventricular function, heterotopic CAVI may be the only available option versus palliation. However, with a preserved right ventricle, all therapies can be considered depending on anatomical assessment by transoesophageal echocardiography and cardiac computed tomography. A coaptation gap of <8.5 mm and a coaptation depth of >10 mm make T-TEER a suitable option, while a large annulus with a coaptation gap of >8.5 mm and a depth of <10 mm will increase the risk of T-TEER failure, thus deeming TV annuloplasty or TTVR more favourable. In cases of a coaptation gap of >8.5 mm and a depth of >10 mm, TTVR is usually a better option, but if the anatomy is not suitable, then CAVI may be considered. In cases of atrial secondary TR, a similar decision-making process is recommended, after discussions with the electrophysiology team regarding whether ablation can be performed prior to TTVI. In patients with primary degenerative TR, transoesophageal echocardiography is also recommended to identify anatomical factors, including coaptation gap and annulus size, and further assess the cause of primary TR. In cases of prolapse or flail, or papillary muscle rupture, T-TEER can be considered. On the other hand, in cases of calcified leaflets or perforation, TTVR is a better option; however, if the anatomical assessment is not favourable, then CAVI can be considered. Finally, in patients with lead-related TR, transoesophageal echocardiography is important to determine whether there is mechanical interaction between the leads and the TV. In those cases, collaboration with the electrophysiology team is valuable to assess whether lead repositioning, extraction or replacement may be helpful. If TR remains an issue, T-TEER, TTVR or CAVI can still be considered depending on anatomical criteria.9
A more simplified algorithm for decision-making was proposed by Fam et al.110 In patients with symptoms of right-sided failure, acceptable right ventricular function and normal pulmonary pressures, both T-TEER and TTVR are reasonable options. In patients with a large annulus and/or coaptation gap, tethered leaflets and/or lead-associated TR, TTVR may be a more favourable option. However, in those with a small coaptation gap, minimal leaflet tethering, right ventricular dysfunction or contraindication to anticoagulation, T-TEER may be a better option.110 In cases of unfavourable anatomy for TTVR, and when T-TEER is not an option, heterotopic CAVI may be considered.70
Conclusion
The emergence of TTVI marks a significant paradigm shift in the treatment landscape for TR and offers hope for many patients who previously had limited options. These less-invasive techniques offer valuable alternatives to traditional surgery. While the initial clinical trials for TTVI devices demonstrated promising results, long-term data on their durability and complication rates remain limited. Navigating this exciting frontier necessitates a balanced perspective that emphasizes multidisciplinary heart team discussions, cost considerations and the importance of patient education. Continued research and development efforts are essential to refine existing devices and explore new technologies to make the procedures relatively easier and shorter to ensure broader applicability and advance the field forward.











