Forty-seven years ago, Andreas Gruentzig introduced ‘percutaneous transluminal coronary angioplasty’ (PTCA) as a new approach to treating symptomatic occlusive coronary artery disease.1Expand Reference The mechanism of balloon angioplasty (BA) formed the foundation around which newer equipment and technologies were developed, including plain old balloon angioplasty (POBA), bare-metal stents (BMS), drug-eluting stents (DES) and drug-coated balloons (DCBs), each addressing specific risks of earlier technologies.2–4234 Coronary artery dissection is a frequent result of vessel injury caused by balloon dilatation. Intravascular ultrasound (IVUS) studies have demonstrated its presence in 50–80% of procedures.5,656 With the return of BA with DCB technology, dissection is now frequently accepted to achieve a ‘DCB-only’ approach. Having stents on the shelf, there is room for aggressive yet controlled lesion preparation. However, coronary dissections remain the most common reason for bailout stenting (BOS) in various DCB studies.7–1078910 Moreover, the outdated National Heart, Lung and Blood Institute (NHLBI) angiographic classification is still in use and, notably, has not been validated or updated recently.11Expand Reference
During the POBA era, coronary dissection leading to acute or subacute abrupt closure was the most feared complication.12Expand Reference Efforts were made to identify clinical and angiographic predictors to prevent and manage such dissections. The purpose of this article is to review the historical background, studies defining clinical, angiographic and morphological patterns of the dissection spectrum and various currently evolving management strategies.
Pre-stent era 1977–1990
Historical background of coronary dissections
Coronary dissection: An inherent risk of balloon angioplasty
History was made on 16 September 1977 when Andreas Gruentzig performed the first transluminal balloon catheter inflation of a discrete left anterior descending artery stenosis in a 38-year-old male patient using a modified, non-steerable balloon dilation catheter.1Expand Reference When the results of this novel innovation were presented at the American Heart Association meeting in November 1977, they were well received and resulted in wider adoption.11Expand Reference In March 1979, the Cardiac Diseases Branch of the NHLBI began centrally accumulating baseline and follow-up data to gain knowledge of the acute and long-term results of PTCA.11Expand Reference According to the PTCA Manual of Operations, ‘coronary intimal dissection (intimal tear) was defined as the presence of angiographically evident intimal damage producing either an intraluminal filling defect or extraluminal extravasation of contrast material; coronary dissection was considered a complication of PTCA if it caused major luminal obstruction or was associated with coronary occlusion, myocardial infarction (MI) or deterioration of flow necessitating emergency coronary artery bypass graft (CABG)’.13Expand Reference
The 1983 complication report of the NHLBI PTCA registry showed an overall rate of 9.4% intimal tear or coronary dissection in the initial 1977–1981 cohort of 1,500 patients from 73 participating centres, with 31% of these dissections leading to major complications of MI, CABG or death.13Expand Reference With an unpredictable occurrence, coronary dissection was recognized as the leading cause of abrupt vessel closure and became the most common indication for emergency surgery, which also carried high early morbidity and mortality risks.14–16141516 With substantial technological advances and more refined tools, the success rates and indications for PTCA expanded exponentially, even in more complex and high-risk patients.11Expand Reference Despite improved efficacy, the incidence of complicated dissections remained unchanged in the 1985–1986 NHLBI cohort.17Expand Reference Meanwhile, concerns were also raised that intimal dissections accelerated early restenosis.18–20181920
The credibility of the novel technology was challenged due to the limited means of managing acute vessel complications, high restenosis rates and their associated costs13–171314151617. In recognition of the incidence of unavoidable dissections, research efforts continued for many years to study the pathophysiology and clinical and angiographic risk predictors and define the precise relationship between intimal dissection and restenosis.12,20–2212202122 Management strategies also evolved from emergency CABG to various repeat angioplasty techniques during early POBA years and subsequently to BOS.
Pathomorphology of coronary dissections
Coronary dissections under microscopy
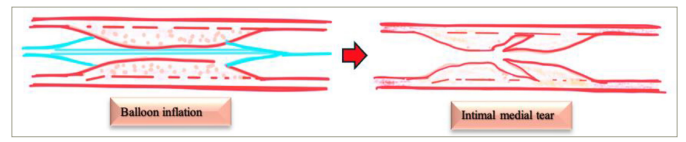
BA results in dilatation of the vascular lumen, with the underlying mechanism attributed to the redistribution and compression of atheromatous plaque, as proposed by Dotter and Judkins, and Gruentzig; however, this has never been proven.23,242324 The exact mechanism remained ill-defined, with various theories being proposed. In 1981, Block et al. described plaque splitting at its thinnest portion in two patients following BA.25Expand Reference In 1983, Waller et al. studied early histological changes occurring from 4 h to 30 days post-BA in several patients.16Expand Reference26Expand Reference The following series of possible mechanisms were reported: intimal tears, cracks or fractures with variable degrees of localized or extensive medial penetrations; intimal–medial dissections propagating either antegrade, retrograde or both directions; and, sometimes, lifting of the plaque from the deep medial layer.26Expand Reference Deep extensive medial dissections may result in a propagating intramural haematoma, subsequently occluding the lumen. The extensive intimal–medial dissection plane may lift the plaque from the media, creating a large flap that eventually curls up into the lumen, causing abrupt vessel closure. Similar patterns of intimal or medial splitting, plaque fractures and haemorrhage were described in autopsy studies.27,282728
Coronary dissections under angiography
The terms ‘intimal tears or flaps’ and ‘dissection’ were used freely to describe the spectrum of morphological alterations on angiography.29–31293031 However, in reality, the result may give diverse angiographic findings, often dictated by the lesion characteristics.30,323032 To classify the angiographic appearances following PTCA, Holmes et al. first described four distinct patterns of immediate angiographic changes in 100 patients, namely, smooth-walled dilatation, intimal flaps or intramural split or dissection, intraluminal haziness and no change in the lesion.29Expand Reference However, there was no anatomical correlation.29Expand Reference Dorros et al. showed safe healing of intimal dissections following PTCA.33Expand Reference Further analysis of the early PTCA registry revealed that about two-thirds of the angiographically detected dissections (9.2%) had a benign course post-PTCA.13Expand Reference The NHLBI PTCA registry concluded that angiographic patterns of intraluminal filling defect, linear luminal density or staining and extravasation of contrast with good distal flow are called coronary intimal dissection.14Expand Reference When a complicated dissection occurs, it is rapidly observed as well-defined, long intramural contrast channels, large radiolucent spiral tracts with persistence of contrast material, an irregular lumen with contrast hang-up, delayed flow and abrupt closure. Subsequently, Dorros et al. devised the widely used NHLBI angiographic classification of dissections (types A–F) in 1985 based on the cine-loop fluoroscopy images of patients from the PTCA registry.13Expand Reference Clinically, types A and B and stable type C were classified as uncomplicated, whereas type C with suboptimal haemodynamic results and types D and F represent complicated dissection. Type E could be a combination of dissection with thrombus.
This system is useful in providing distinct angiographic categories as shown in Table 1, but its application was limited during the late POBA era (Figure 1).
Table 1: NHLBI classification of types of coronary artery dissections during angioplasty
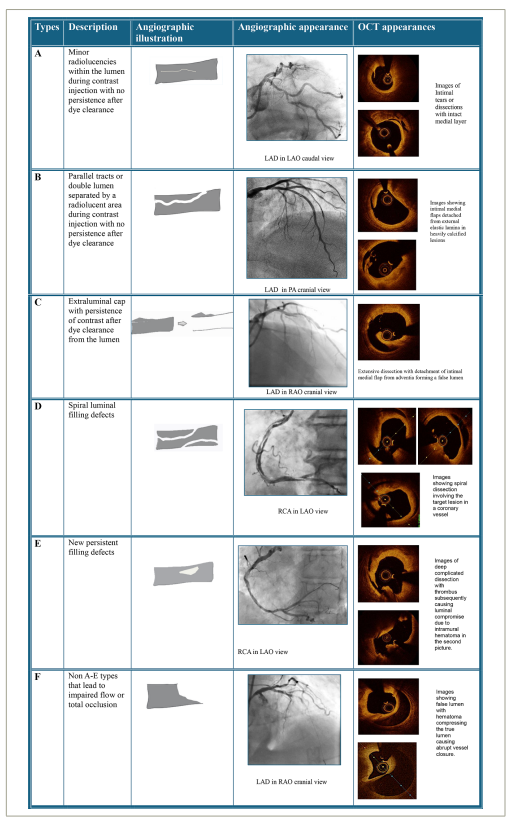
Types A- F refers to NHLBI classification types of dissections. OCT images in the fifth column depict the counterparts of each NHLBI dissection types and are not the actual OCT runs of the angiogram images shown in column 4.
LAD = left anterior descending artery; LAO = left anterior oblique view; NHLBI = National Heart, Lung and Blood Institute; OCT = optical coherence tomography; PA = posteroanterior; RAO = right anterior oblique view; RCA = right coronary artery.
Figure 1: Mechanism of balloon angioplasty
Angiographic–morphological correlations
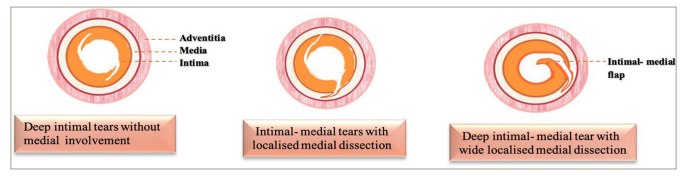
The correlation between angiographic appearances and the morphological patterns of BA mechanisms was well described by Bruce F Waller in 1988.21Expand Reference He examined histopathological specimens from 76 coronary artery segments containing angioplasty sites from 66 patients with necropsy who died within 30 days of PTCA, comparing the angiographic description by different PTCA operators with the anatomical findings. Interestingly, angiographic ‘intimal flaps’ (43%) and ‘intraluminal haziness’ (38%) mostly correlated with intimal–medial splits or cracks of varying degrees, accompanied by localized medial dissection. Four extensive medial dissections (9%) were seen in the intimal flap category, while the haziness pattern had a mix of pure intimal injuries (31%) and laminated thrombus coating (3%). At a ‘coronary artery dissection’ site, a deep intimal–medial tear (Figure 2) with an extensive longitudinal medial dissection had occurred. The adventitial extension was seen in two patients, where the ‘extravasated contrast’ material correlated to confined coronary perforation (confined rupture). In his later works, there was evidence of regression of these intimal flaps, with no histological signs of previous vessel injury.30Expand Reference
Figure 2: Diagram showing morphological correlates of angiographic appearances of intraluminal flaps and haziness
A morphologic–angiographic–clinical nomenclature of dissections
Histopathological studies added to the existing angiographic terms by providing an anatomical perspective. Histologically, dissection was defined as penetration into the medial layer. Angiographic coronary artery dissection describes a visible intimal flap (anatomically equivalent to intimal–medial tear), with contrast staining extending beyond the confines of the angioplasty lesion, with or without clinical symptoms or signs of ischaemia.30Expand Reference Such intimal flaps could either extend circumferentially in the short-axis view or propagate in anterograde or retrograde directions longitudinally in the long-axis plane, as shown in Figure 3. By using different angiographic projections, an estimate of the biplanar extent is possible. Theoretically, a dissection involving >50% of short-axis circumference or >1 cm of anterograde or retrograde of long-axis length was defined as a complication of angioplasty, whereas anything below these cut-offs were mechanisms. On the contrary, intimal dissection or split described an intimal flap with contrast staining with no evidence of ischaemia.30,313031
Figure 3: Morphological correlation of angiographic appearances of complicated coronary arterial dissection
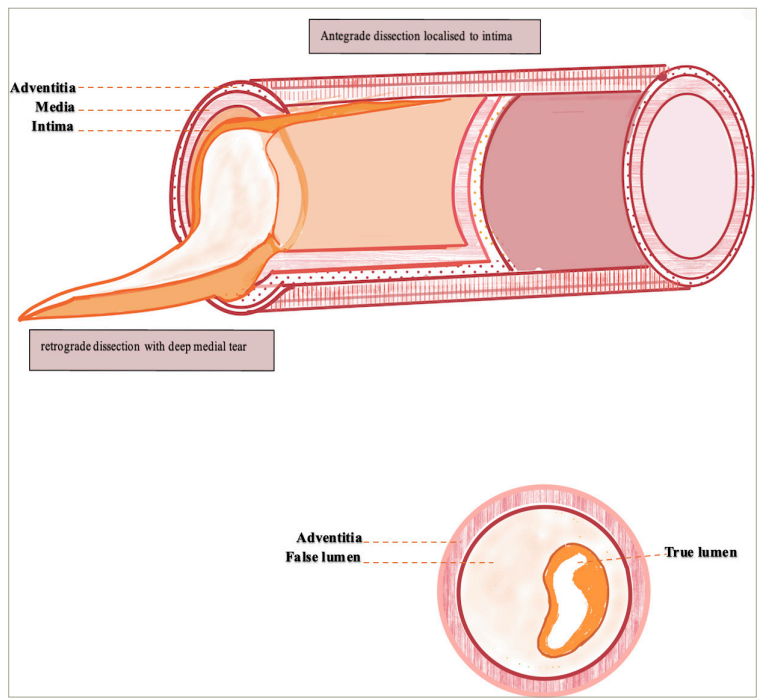
Diagram showing morphological correlation of angiographic appearances of complicated coronary arterial dissection, caused by deep extensive medial dissection creating a false lumen and compressing the true lumen
Therapeutic dissections: Uncomplicated and safe-to-leave category
During the early years of angioplasty, concerns were raised that intimal tears or dissections accelerate early restenosis.18–20181920 To address this, in 1985, Leimgruger et al. studied the haemodynamic significance of uncomplicated angiographic coronary intimal dissections by measuring transtenotic pressure gradient following successful PTCA and examining their relationship with restenosis by using a validated digital electronic caliper method to measure diameter stenosis (DS) severity.22,342234 A transtenotic pressure gradient was obtained using a guide catheter, guidewire and a balloon catheter. By positioning the guidewire and balloon catheter across the coronary stenosis, a pressure port distal to the balloon segment recorded the distal arterial pressure, while the guiding catheter tip at the ostium monitored the proximal arterial pressure. The difference between these phasic pressures yielded the transtenotic pressure gradient using a specialized computer program. Using this technology, the authors demonstrated that such dissections did not increase the risk of restenosis and had a beneficial effect of lowering the restenosis rate if the final transtenotic pressure was ≤15 mm Hg. In fact, if the final gradient was >15 mm Hg, the rate of restenosis was not significantly different between the groups with and without intimal dissections (35 versus 39%; p=non-significant). Matthews et al. then showed that patients with dissections during PTCA are unlikely to develop restenosis at 1-year follow-up.35Expand Reference In this observational study of 273 patients, 82% of the dissection group did not develop restenosis. Similar retrospective studies during the same year showed a similar relationship between intimal dissection and restenosis.35–40353637383940 The term ‘therapeutic dissection’ was widely used to describe uncomplicated coronary intimal dissections that resulted in an increased cross-sectional area (CSA) and were less likely to develop restenosis.40,414041 Table 2 summarizes studies examining the relationship between lesions with or without dissections and restenosis.22,32,35,42–4522323542434445
Table 2: Summary of studies that examined the relationship between lesions with or without dissections and restenosis22,32,35,42–4522323542434445
|
Studies |
Year |
Patients |
Angiogram f/u (%) |
Dissection (% of lesions) |
Restenosis at f/u (%) (with versus without dissections) |
p value |
|
Leimgruber et al.22Expand Reference |
1985 |
1,650 |
60 |
25ϕ |
19 versus 28 gradient ≤15 |
<0.05 |
|
Matthews et al.35Expand Reference |
1988 |
216 |
30 |
34 |
18 versus 23 |
NS |
|
Black et al.32Expand Reference |
1988 |
384 |
39 |
34 |
29 versus 32 |
NS |
|
Quigley et al.42Expand Reference |
1989 |
114 |
88 |
20 |
35 versus 31 |
NS |
|
Renkin et al.43Expand Reference |
1990 |
278 |
47 |
33 |
38 versus 31 |
NS |
|
Bourassa et al.44Expand Reference |
1991 |
307 |
80 |
41 |
33 versus 36 |
NS |
|
Hirshfeld et al.45Expand Reference |
1991 |
694 |
73 |
39 |
40 versus 39 |
NS |
ϕ = uncomplicated dissections; Angiogram f/u = per cent of patients with angiographic follow-up; f/u = follow-up; gradient = final transtenotic pressure gradient in mm Hg; NS = not significant.
Complicated dissections: Indeterminate category and ‘need-to-graft’ category
As described earlier, complications occur when an intimal–medial tear produces a flap that could fold or become free in the lumen, collapsing on itself and causing intussusception, which compromises the lumen.21Expand Reference Tissue disruption may produce turbulence (shear stress), stasis and thrombosis, leading to suboptimal haemodynamic results.46,474647 If intervened promptly in suitable cases, the flaps can be made to adhere to the vessel wall through further dilatation techniques, thereby restoring distal flow.48Expand Reference If the flow is compromised despite rescue strategies, the earliest mode of definitive therapy is emergency surgery, as explained below.
In essence, the challenge lies in interpreting dissections via angiography. When intimal damage exposes the thrombogenic plaque layers, contrast fills these furrows during further injections. Angiography reveals hazy or ill-defined margins of an enlarged lumen, with inhomogeneous opacification, double-line or contrast filling defects. Regardless of the mechanism, not all the dissections are visible, and not all visible dissections are complicated. Sometimes, a combination of angiographically undetectable dissection, recoil, refractory spasm and intracoronary thrombus can occur. This is a conundrum still faced today, where an indeterminate angiographic appearance must be classified as either safe or unsafe. Hence, safe dissections may be viewed as a therapeutic mechanism of BA, while unsafe dissections represent a complication of dilatation.
Existing classification systems
NHLBI angiographic classification of dissections-1985
Types A–F, as shown in Table 1, represent angiographic appearances of the contrast in relation to their clearance and their effect on distal flow, as described in 1985.49Expand Reference Neither the circumference nor the length was factored in. It was only in 1991 that Huber et al. retrospectively predicted the clinical outcomes of dissections using NHLBI classification.39Expand Reference Of 691 dissections, 543 were type B, which had no higher risk of morbidity and mortality compared with patients with no dissections. A small subgroup of types C–F (n=148) had a statistically significant increase in in-hospital complications against type B. The results are severely limited by the low power of the subgroup and unreported inter- and intraobserver variability. In contrast, slightly better clinical outcomes were observed for types B–F dissections in an unblinded MERCATOR (Multicentre European Research trial with Cilazapril After angioplasty to prevent Transluminal coronary Obstruction and Restenosis) trial by Hermans et al. in 1992.40Expand Reference As shown in Table 3, there is no significant difference in long-term events between the different NHLBI types of dissections.39,40,50394050 While it is difficult to draw any strong conclusion from these studies with conflicting results, the concept of therapeutic dissections (roughly types A–C, some type Es if the thrombus clears) has been strengthened. Nevertheless, the decision to treat a dissection depends on the clinical and haemodynamic parameters in relation to distal perfusion, and this approach still applies in current practice.
Table 3: Summary of studies that examined the relationship between lesions with dissections, NHLBI types and their outcomes39,40,50394050
|
Studies |
Year |
Patients |
NHLBI dissection types with number of dissections (%) |
NHLBI dissection types with acute complications, n (%) |
NHLBI dissection types with late events, n (%) |
|
Huber et al.39Expand Reference |
1991 |
691 |
B: 543 (78.6) C: 62 (9) D: 33 (4.8) E: 18 (2.6) F: 35 (5.1) |
B: 17 (3.1) C: 6 (9.7) D: 10 (30.3) E: 7 (38.9) F: 24 (68.6) |
NR |
|
Hermans et al.40Expand Reference |
1992 |
693 |
247 (32) NHLBI A: 76 (11) B: 136 (19.6) C: 33 (4.8) D: 3 (0.4) E: 3 (0.4) F: 1 (0.1) |
NR |
TLR A: 12 (15.8) B: 18 (13.2) C: 2 (6.1) D–F: 0 |
|
Albertal et al.50Expand Reference |
2001 |
256 |
A and B: 100 C: 32 |
A and B: 3 (2) C: 1 (2) |
A–B: 11 (11) C: 4 (13) |
Acute complications included abrupt closure, Q wave myocardial infarction and emergency and elective coronary artery bypass. Late events included revascularization.
NHLBI = National Heart, Lung and Blood Institute; NR = not reported; TLR = target lesion revascularization.
In 2001, Albertal et al. showed that moderate dissections (as classified in Table 4), when left unstented, had good outcome with a classification based on clinical relevance.50Expand Reference
Table 4: Evaluation of dissections by Albertal et al. based on clinical parameters50Expand Reference
|
Grading of dissections |
|
“mild” dissections (type A*or B*), |
|
“moderate” dissections (type C* without signs or symptoms of ischaemia), |
|
“severe” dissections (type C* with symptoms or signs of ischaemia plus types D* to F*). |
|
*Type A-F refers to NHBLI system of classification. |
NHLBI = National Heart, Lung and Blood Institute.
Various forms of classification of dissections have been reported in studies throughout the years of POBA and the early stent era, mainly based on the operator’s experience and preference. While some used mild, moderate and severe dissections, few preferred intimal and coronary arterial dissections.32Expand Reference Given the limitations of the NHLBI angiographic classification, newer classification systems using IVUS or angioscopy modalities were proposed to correlate plaque characteristics with dissection risk.51–53515253
Intravascular ultrasound patterns of dissections
During the stent era, IVUS was increasingly used to show real-time cross-sectional observation of the vessel response to BA and stenting. IVUS gave an in-depth assessment of lesion morphology, and the results of BA with IVUS imaging were consistent with Waller’s histopathological studies.5Expand Reference This helped in deciding the interventional strategies in the event of suboptimal dilatation according to the plaque composition, calcification and eccentricity. In 1992, Honye et al. and Gerber et al. simultaneously published their IVUS experiences with dissections, suggesting a similar classification system.5,51551 Honye’s classification method failed to have good correlation with angiography detected dissections, as 10 out of 23 angiographic dissections in his study were not seen on ultrasound. On the other hand, Gerber’s dissection patterns were very detailed but proved complicated and time-consuming for operators with basic IVUS interpretation skills. The application of this technology declined in the field of dissections due to a number of reasons. First, IVUS failed to detect dissection flaps that adhered to the wall when it transversed beyond them.54Expand Reference Second, it poorly differentiated the echo-free space of thin diseased media from dissection planes. Finally, IVUS-detected severity of dissections did not correlate with any pre-interventional lesion characteristics.54Expand Reference Figure 4 illustrates IVUS appearances of coronary dissections during percutaneous coronary intervention (PCI).
Figure 4: Intravascular ultrasound images of coronary dissections during percutaneous coronary intervention
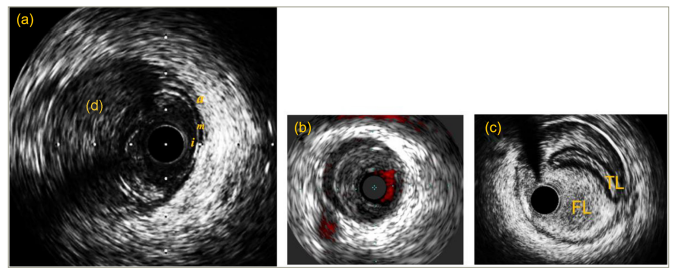
(a) Echogenic intramural haematoma (d) seen in the dissection plane. (b) Chromoflo IVUS image showing an echo-free space representing a false lumen. (c) A large false lumen compromising the true lumen during PCI as a result of guide-induced dissection and IVUS confirms that the wire is in the false lumen
a = adventitia; d = dissection; FL = false lumen; i = intima; IVUS = intravascular ultrasound; m = media; PCI = percutaneous coronary intervention; TL = true lumen
Intravascular ultrasound validation of therapeutic dissection concept
However, IVUS technology continued to improve with high-resolution and low-profile catheters. In 2000, Schroeder et al. demonstrated that IVUS-detected therapeutic dissections (mild and moderate group) did not impact acute or long-term outcomes, further substantiating the concept of therapeutic dissections.52 Expand ReferenceSchroeder’s classification method was rather simple and easy to use, as in Table 5.
Table 5: Schroeder’s intravascular ultrasound dissection criteria52Expand Reference
|
(a) Mild dissection with the presence of a partial tear |
|
(b) Medium dissection with a tear through the plaque (50% plaque diameter) |
|
(c) Severe dissections with a second channel extending into the media with a clearly identifiable second lumen after contrast dye |
Following this study, in 2001, Shigeyama et al. attempted to classify the therapeutic dissections category based on angiography into types A–E in relation to the depth and breadth of dissection and the presence of intimal flap or spiral appearance.53Expand Reference However, its clinical application is limited, as the interest was more in the management of indeterminant groups of dissections.
Optical coherence tomography: A better lens for drug-coated balloon-related dissections?
Since 1991, the use of optical coherence tomography (OCT) has expanded rapidly and is now a preferred modality for precisely imaging coronary luminal architecture, differentiating plaque rupture or erosion, vulnerable plaque identification and dissections.55Expand Reference OCT has been considered safe for imaging spontaneous coronary artery dissections, although some clinical risk has been reported.56–6256575859606162 The superior spatial resolution of OCT identifies intramural haematoma, endothelial tears or entry sites of dissection.55,635563 Given the low clinical risk, OCT remains indicated in cases of dissection with diagnostic uncertainty.57,59,60575960 An OCT-guided DCB strategy is an area of interest in recent DCB studies, with reconstruction software allowing accurate quantification of dissection depth and volume, as shown in Figure 5.64–66646566 A recent study called TRANSFORM (TReAtmeNt of Small Coronary Vessels: MagicTouch Sirolimus Coated Balloon; ClinicalTrials.gov identifier: NCT03913832) demonstrated that OCT-derived absolute dissection volume had a favourable effect on lumen gain post-DCB in the paclitaxel DCB arm compared with sirolimus arm in small de novo coronary vessels.64Expand Reference Furthermore, OCT fused with angiography provides a realistic reconstruction of lumen architecture with vessel wall dissections, guiding operators to formulate a specialized treatment for patient subsets with DCB-related dissections.67Expand Reference This could be the future of DCB technology, with further research in this field currently underway.
Figure 5: Measurement of dissection area by optical coherence tomography (QCU-CMS)64Expand Reference
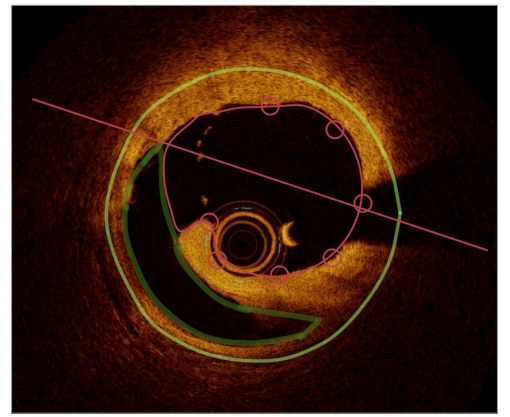
A straight line connecting the edges of the flap of dissection isolates the dissection space from the lumen. The volume of the dissection is calculated by Simpson’s rule over the entire length of the dissection
QCU-CMS = Quantitative Coronary Ultrasound-Clinical Measurement Solution
Coronary dissections and risk factors
Angiographic predictors of dissection risk and its sequelae play a vital role in devising management strategies. In this article, we review the studies identifying clinical and angiographic risk factors, with Table 6 illustrating such associations.12,13,17,22,32,40,68–71121317223240...71
Table 6: Factors associated with coronary dissections12,13,17,22,32,40,68–71121317223240...71
|
Clinical |
|
Age ≥62 years40Expand Reference |
|
Female gender13,221322 |
|
Acute coronary syndromes32,68,69326869 |
|
Low cholesterol <5.7 mmol/L40Expand Reference |
|
Angiographic |
|
RCA lesion12,13,17,4012131740 |
|
Multivessel disease68–70686970 |
|
Localization at a bifurcation or a curve12,401240 |
|
Length of the lesion69–71697071 |
|
Diffuse disease69Expand Reference |
|
Eccentric stenosis12,40,69,7112406971 |
|
Irregular borders12,321232 |
|
Intraluminal lucency*12,401240 |
|
Procedural |
|
Larger balloon assignment (>1.3:1)69,706970 |
|
Higher inflation pressure40Expand Reference |
|
Multiple lesion dilatation69,706970 |
|
Multisite dilatation69,706970 |
|
Dilatation at a tortuosity12,401240 |
*Intraluminal lucency is a correlate of plaque rupture, ulceration, subintimal haemorrhage or superimposed or recanalized thrombus.
RCA = right coronary artery.
In NHLBI cohorts, intimal dissections were associated with female gender, right coronary artery lesions, multivessel disease, and eccentric and diffuse disease.17Expand Reference In 1986, Ischinger et al. identified complicated angiographic characteristics – such as irregular borders, intraluminal lucency and stenosis located at a bifurcation or a curve – as predictors of dissections.12Expand Reference In 1985, Bredlau et al. showed that the strongest predictor of a major ischaemic complication was the procedural appearance of an intimal dissection, with a 6.5-fold increase in risk of MI, emergency CABG and death.72Expand Reference Later, in a haemodynamic study in 1987, Redd et al. first graded the degree of disruption angiographically into intimal (when the luminal or extraluminal contrast staining remained within the confines of the original PTCA lesion) and arterial dissections (when extending beyond the lesion either proximally or distally).73Expand Reference The authors studied the relationship between the dynamic behaviour of the transtenotic pressure gradient after each balloon inflations and the presence of disruptions with subsequent vessel closure. Patients with a rising trend in transtenotic pressure gradient had a higher incidence of arterial dissections (25 versus 7%; odds ratio [OR] 4.8; p=0.001) but not the isolated intimal tears alone.73Expand Reference Multivariate analysis of procedural variables showed that a rising transtenotic gradient trend (OR 1.99; p=0.002), lesion length (OR 1.11; p=0.007) and post-PTCA gradient (OR 1.06; p=0.001) were strong predictors of arterial dissections. The rising trend in transtenotic pressure was significantly associated with other ischaemic complications, such as acute closure (OR 2.04; p<0.001), CABG (OR 1.13; p<0.001) and MI (OR 2.91; p<0.001). Additionally, in 1989, Black et al. analysed the morphological variables, dissection length, DS of ≥25% following dilatation and video-densitometry assessment of luminal CSA of <2 mm² and were strong correlates of arterial dissection with ischaemic complications.32Expand Reference The extraluminal contrast cap had a slightly weak correlation compared with the rest of the variables.
There was mounting evidence that intimal dissections were therapeutic. PTCA operators became more aware of the safety of uncomplicated intimal dissections, and as their experience expanded, the interest in salvaging the indeterminant and complicated dissection group led to a strategy of lesion-specific device therapy to avoid abrupt vessel closure and emergency surgery.
Management of coronary dissections: Early and late plain old balloon angioplasty era
In the early era of POBA, any dissections causing acute coronary occlusion were treated surgically. Despite prompt surgical revascularization, more than 50% of patients developed significant MI due to the unavoidable delay of sternotomy. Notably, vein grafts, rather than the left internal mammary artery (LIMA), were used for expediency.74Expand Reference Subsequently in an effort to reverse abrupt vessel closure non-surgically, attempts were made to reopen the occluded vessel by relieving spasm and thrombus pharmacologically, using intracoronary vasodilators, thrombolytics and heparin infusions.75,767576 When these measures were exhausted and MI was imminent, intra-aortic balloon pumps were used to limit myocardial injury before vein grafting.76Expand Reference Reperfusion catheters were used in 1986 to allow optimal bypass grafting.77,787778 However, in the absence of chest pain and acute electrocardiogram changes, even large, flow-limiting dissections were treated with semi-elective bypass surgery. A variety of PCI management options were also subsequently devised to deal with unsafe dissections.
Repeat percutaneous transluminal coronary angioplasty redilatation technique
Immediate repeat dilatation and successful reopening of the occluded dissection in seven patients was first reported in 1984 by Marquis et al.79Expand Reference This approach became routine for treating dissections complicating abrupt reclosures during or after PTCA in following years. About 50% of patients had successful restoration of antegrade flow, thereby avoiding extensive myocardial damage and emergency surgery.12,80,81128081
Tack-back technique
Further technical improvization was made by using a standard balloon of the same or slightly larger diameter and performing low-pressure inflations at increments of one or two atmospheres for 60–180 s repeatedly to gain patency.48,68,81,8248688182 In theory, this remodelled the lesion by ‘tacking up’ the dissected flap and stabilizing dissections with high success rates.83Expand Reference The successful tack-back phenomenon restores a patent lumen possibly by allowing the tissue flaps to adhere to the damaged vessel wall. In an analysis of 109 patients, Lincoff et al. demonstrated that prolonged balloon inflation was an independent correlate of successful resolution of vessel closures (OR 5.11; p=0.001) on multivariate analysis.83Expand Reference
Prolonged balloon inflation using auto-perfusion catheters
When repeat BA failed, prolonged balloon inflations were undertaken with the aid of an auto-perfusion catheter.84Expand Reference A specialized large profile Stack haemoperfusion catheter was first used in 1988, maintaining distal vessel perfusion through the proximal and distal catheter holes, simultaneously facilitating prolonged inflations.85Expand Reference The inflation durations were 3–30 min depending on the tolerance of the patient. It proved very effective in improving outcomes in PTCA refractory dissections, but its use was limited due to passive inadequate perfusion, unfavourable coronary anatomy (side branch occlusion, small vessel and tortuosity), poor guidewire access to the distal vessel, difficult delivery and the advent of better techniques.86–908687888990 The Ringer perfusion balloon catheter (Ringer PTCA) is a rapid-exchange 0.014″ compatible balloon catheter that conforms into a helical cylinder upon inflation and maintains distal perfusion flow, as shown in.91Expand Reference A prospective, multicentre, single-arm clinical study of 60 patients demonstrated that the balloon was well tolerated in the majority of patients susceptible to procedural ischaemia when inflated for 60 s or more.92Expand Reference The Food and Drug Administration recently approved its use in the USA, and it is mainly indicated in PTCA and bypass grafts.93,949394 This technology could potentially be used in scenarios involving indeterminate dissections that necessitate modification.
Controlled inflation technique
Progressive coronary dilation, which involves predilating the stenosis with a smaller balloon followed by maximal dilation with an optimally sized balloon, produces less uncontrolled injury and, thus, reduces the incidence of major complications. This was demonstrated by Banka et al. in a study consisting of 1,486 vessels.95Expand Reference The success rate with this technique was 98.7% in 1,248 partially occluded vessels and 88% in 353 totally occluded vessels. This technique markedly lowered the incidence of acute closure, major dissection, emergency coronary bypass and death in that dilation of both simple and complex lesions.95Expand Reference
Directional coronary atherectomy and balloon pyroplasty
Resection of occlusive dissection flaps causing luminal compromise using Atherocath devices (Devices for Vascular Intervention [DVI], Redwood City, CA, USA) was reported in a few cases with success rates of around 80% during the early 1990s.96–9996979899 Directional coronary atherectomy did not gain much popularity due to the greater risks of vessel perforation, inconsistent results and technical difficulties. Sealing of dissection flaps by imparting various forms of thermal energies, such as laser, radio-frequency and microwave, had been used in the past around 1990s but remained academic due to the risks of restenosis and cost.100–104100101102103104
Bailout stenting
In 1987, intracoronary stainless-steel stents were described to address abrupt closure, and in subsequent years, they were shown to reduce restenosis.2Expand Reference Sigwart et al. demonstrated the first emergency implantation of the endoluminal Wallstent (Schneider, Inc, Minneapolis, MN, USA) for acute occlusion caused by dissection in 13 patients in 1988.105Expand Reference Stents were effective in achieving better angiographic appearances of intimal dissections by securing the flaps and increasing residual lumen diameter.106Expand Reference Gianturco-Rubin Palmaz-Schatz stents, along with other varieties of BMS, became very popular in handling bailout situations and reducing the incidence of Q-wave MI and emergency CABG.83,107,10883107108 However, a multitude of thrombotic, bleeding and restenosis risks then ensued with acute and subacute stent thrombosis emerging as a problem.109–111109110111 When compared with auto-perfusion BA for acute closure in a non-randomized trial, the stent group had a higher subacute reclosure rate and more deaths.112,113112113 Emergency CABG was still required when large dissections could not be repaired, the bailout methods failed or perforation occurred, and in the emergency settings, the conduit choice slowly shifted to left internal artery grafts from saphenous veins.74,11474114
Subsequently, stent technology underwent many technical advancements from using heparin-coated thick bare-mounted rigid coils to the ultrathin DESs used currently, yet there are persistent risks of restenosis, thrombosis and stent failures.115,116115116
Drug-coated balloon era
The concept of DCB angioplasty is ‘device-mediated drug delivery’ to a target lesion using a conventional semi-compliant balloon coated with an antiproliferative drug.4Expand Reference Prior to DCB delivery, the target lesion must be adequately prepared to achieve an acceptable acute lumen gain and to identify lesions prone to acute vessel closure and dissection.117Expand Reference In the event of flow-limiting, vessel-threatening dissections and >30% residual stenosis after extensive and optimal lesion preparation, BOS is recommended.8Expand Reference The rates of BOS across major DCB studies are around 5–22%, and high-grade coronary dissection remains the predominant indication for BOS, alongside acute vessel recoil.7,8,118–12278118119120121122 While the DCB expert consensus document recommends BOS for any dissections equivalent to or greater than type C in the NHLBI angiographic classification, there are studies that have shown that non-flow-limiting moderate dissections, including type C, are safe when left alone.8,50,123850123
Severe dissections (types D and F NHLBI) are universally treated as a complication that requires stent deployment to prevent periprocedural MI. The management of mild-to-moderate dissections (types A–C) generally varies among interventionalists, based on their experience in DCB angioplasty. In the past two decades of the DES era, the vast majority of lesions undergoing PCI are stented.
However, the threshold to consider BOS in cases of dissections will become higher with increasing experience in DCB angioplasty when a refined lesion preparation algorithm is applied.
Conclusion
Coronary dissections are a stumbling block to the widespread adoption of ‘DCB-only’ angioplasty, and this limitation can be overcome with the lessons from the POBA era and a change in outlook towards conservative management of coronary dissections dictated by the clinical situation and patient safety. Re-learning the avoidance, recognition and management of coronary dissections will facilitate an increased uptake in this promising new PCI concept of ‘leave nothing behind’.













