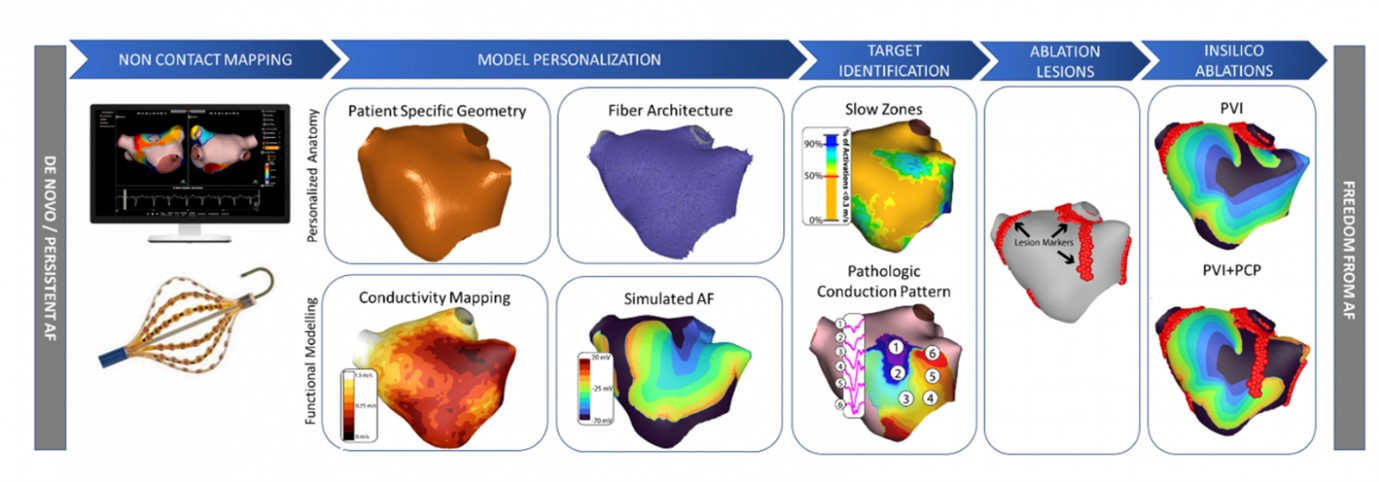
Background: Improving outcomes with ablation of non-paroxysmal AF has proved challenging because of large interindividual variability in the underlying electrical and anatomical substrate. Computational models personalized to patient electroanatomic mapping (EAM) data can provide an inexpensive technology to predict outcomes for non-PV ablation strategies in terms of success, failure or reduced AF complexity on a patient-specific basis. Performance of non-contact charge density mapping to guide ablation of non-PV targets in PsAF following either a first or second failed procedure was evaluated in a recent multicentre trial, RECOVER AF, and showed encouraging outcomes. This study aims to assess the predictive potential of LA computational models personalized to PsAF patient data for simulating the outcome of sequential ablation strategies outside of PV isolation.
Model pipeline: EAM data from cases of an on-going observational, prospective, multicentre, multi-national, open-label registry (DISCOVER) were used. Electrophysiology models were personalized to patient anatomic and tissue conduction properties using electroanatomic recordings, shell geometry and histological observations of average fibre-direction in the left atrium. Tissue conduction properties and cellular level dynamics of the pathologic conduction areas were modified based on activation times and conduction velocity heterogeneity obtained through Acutus AcQMap non-contact mapping catheter recordings. Three types (localized partial rotational activity [LPRA], localized irregular activity [LIA] and focal activity) of pathologic conduction pattern (PCP) targets as identified through the non-contact AcQTrack system were simulated to model pathologic propagation using openCARP electrophysiology simulator. Simulations of personalized model were used to assess the effect of PVI, PCP and PVI+PCP ablation lesions in comparison to the baseline AF case.
Results: Personalized model provided a proof-of-concept in silico EAM test bench to predict wave behaviour through a comparison of AF complexity with PVI (marginal or no change) to PVI + PCP ablations (extensive decrease).
Discussion: This study used a stepwise ablation approach in subject-specific computational models to provide a means to guide effective therapy guidance towards optimal patient outcomes. Targeting pathologic propagation identified during AF effectively reduces AF complexity, and potentially improves long-term freedom from AF. ❑
Figure 1: Respiratory pattern before versus after ablation