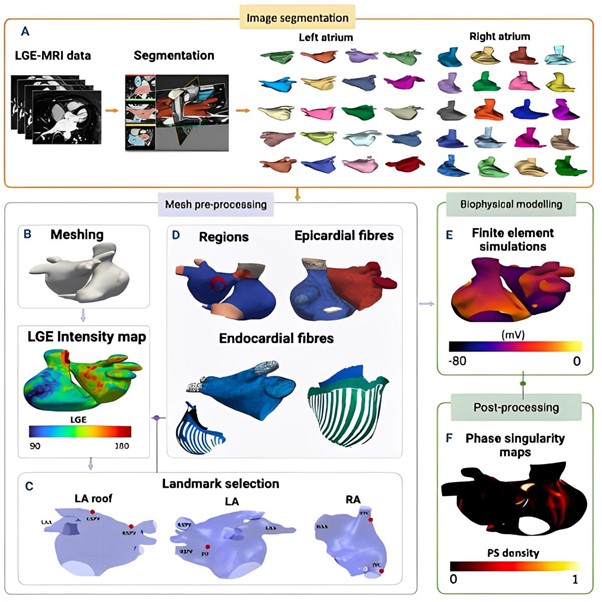
Introduction: Recent clinical and mechanistic modelling studies have identified the significance of the right atrium (RA) in understanding the mechanisms underlying atrial fibrillation (AF) recurrence, though its role for each individual patient remains unclear. Predicting patient-specific response to treatment to guide therapy requires a framework for integrating personalised anatomy, fibrosis, and electrophysiology data. The first aim of this study was to develop an open-source biatrial modelling pipeline to construct personalised biatrial models integrating these data. The second aim was to use these models to assess AF inducibility and predict AF ablation outcomes for an individual patient on clinical timescales.
Methods: Patient-specific models were constructed from late-gadolinium enhanced-MRI scans for a total of 70 patients from the Atrial Segmentation Challenge Dataset (2018). The construction of such models entailed manually delineating atrial substructures (Figure 1A) and pre-processing meshes to quantify scar fibrosis (Figure 1B), followed by landmark selection (Figure 1C) and inclusion of atrial fibres from an atlas (Figure 1D). Each patient-specific model incorporated atrial fibrosis, atrial fibres, structures, and electrical parameters to investigate variances in atrial properties and assess AF inducibility. Finally, finite element simulation stress tests were performed (Figure 1E) and post-processed to evaluate variations in atrial fibrillation wavefront patterns (Figure 1F). Different ablation approaches were simulated across the cohort, including pulmonary vein isolation and fibrosis-based ablation.
Results and discussion: The integration of the right atrial substrate resulted in significant increases in driver activity and in the total number of drivers (indicated by phase singularities) in the posterior region of the left atrium (LA) across the cohort, indicating that AF is more likely to be sustained. The LA had a greater mean phase singularity density of 3.8 phase singularities/cm2 than the RA, indicating that targeting the LA is critical in preventing AF recurrence. The biatrial phase singularity density maps demonstrated a significant increase in localised regions with elevated phase singularity density, indicating regions of re-entry or wavefront break-up.
Conclusion: A model construction pipeline demonstrates that biatrial models have the potential to be efficient tools for predicting AF treatment outcomes and personalising therapy on clinical timescales. We are using our pipeline to compare different ablation approaches and anti-arrhythmic drug therapies. ❑
Figure 1