Introduction: Scar and autonomic remodelling are proposed pathophysiological mechanisms in atrial fibrillation (AF). Autonomic remodelling has been shown to trigger ectopy that initiates AF. In animal models, autonomic remodelling shortens the atrial effective refractory period and wavelength of the cardiac impulse, which in turns increases the probability of multiple re-entrant circuits existing simultaneously and thereby enhance AF maintenance. In humans, it is unclear how autonomic remodelling impacts electrophysiological properties. In this study, the impact of autonomic modulation on CV dynamics and wavefront propagation was evaluated.
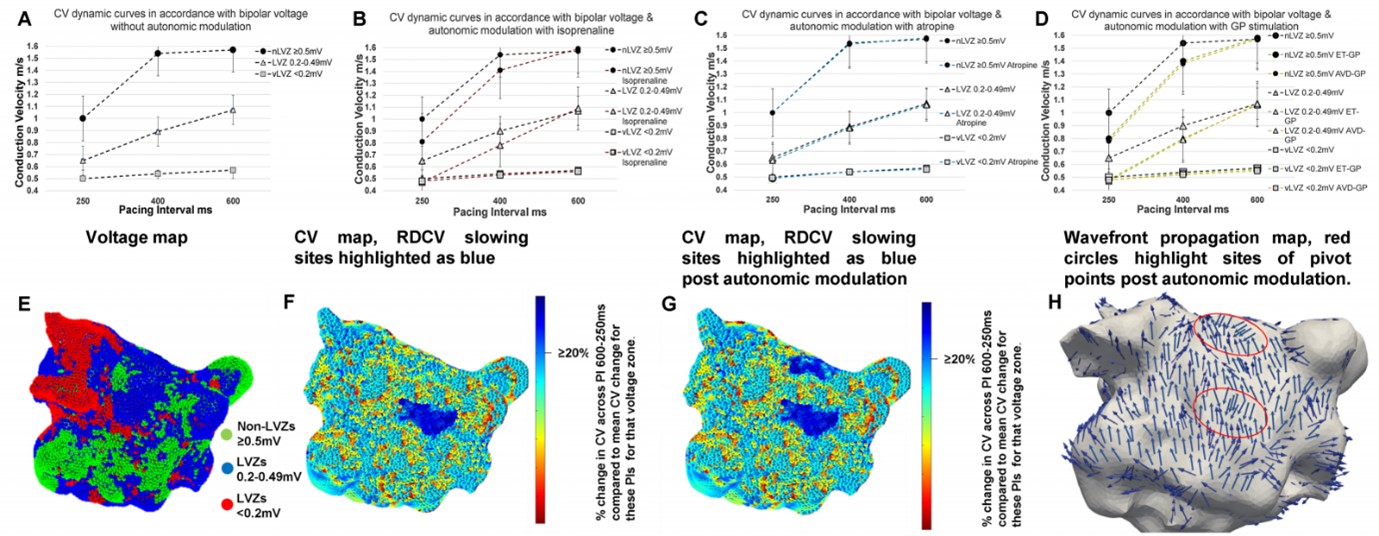
Methods: Local activation times (LATs), voltage and geometry data were obtained from patients undergoing ablation for persistent AF. LATs were obtained at 3 pacing intervals (PI) (600, 400 and 250 ms) sinus rhythm (SR). LATs were used to determine CV dynamics and their relationship to voltage. Sites of enhanced CV heterogeneity was identified in all patients, defined as rate dependent CV (RDCV) slowing sites, which are zones exhibiting a reduction in CV between PI=600 ms and PI=250 ms of ≥20% of the mean CV reduction seen between these PIs for that voltage zone. The impact of autonomic modulation, pharmacologically and with ganglionated plexi (GP) stimulation, on CV dynamics and wavefront propagation was determined i.e., pivot points (change in wavefront propagation of ≥90°).
Results: Fifty-four patients were included. Voltage impacted CV dynamics whereby at nLVZ (≥0.5 mV) the curves are steeper (0.03 ± 0.02 m/s ∆CV PI 600–400 ms [PI1], 0.54 ± 1.1 m/s ∆CV PI 400–250 ms [PI2]), broader at LVZ (0.2–0.49 mV) (0.16 ± 0.09 m/s ∆CV PI1, 0.23 ± 1.1 m/s ∆CV PI2) and flat at vLVZ (<0.2 mV) (0.04 ± 0.01 m/s ∆CV PI1, 0.05 ± 0.02 m/s ∆CV PI2). RDCV slowing sites were identified in all patients, with an average of 2.8 ± 1.1 RDCV slowing sites per patient with a total of 149 RDCV slowing sites. RDCV sites were predominantly seen in LVZs (0.2–0.049 mV) (117/149, 78.5%). No RDCV sites were demonstrated in vLVZs (<0.2) and the remaining 32 (21.5%) RDCV slowing sites were seen in nLVZs (≥0.5 mV). Out of the 54 patients, 24 (44.4%) and 30 (55.6%) patients had CV maps created with autonomic modulation pharmacologically and through GP stimulation respectively. Atropine did not impact CV dynamics, whilst isoprenaline and GP stimulation resulted in enhanced CV slowing with rate (Figure 1A–D). Isoprenaline (2.7 ± 1.1 increase per patient) and GP stimulation (2.8 ± 1.3 increase per patient) resulted in enhancement of CV heterogeneity sites i.e., RDCV slowing sites (Figure 1E–G). Most pivot points co-located to RDCV slowing sites (80.2%). Isoprenaline (1.7 ± 1.2 pivot points per patient) and GP stimulation (1.9 ± 1.1 pivot points per patient) also enhanced the number of pivot points identified. Figure 1H demonstrates two pivot points (red circle) seen post autonomic modulation of which only one was present without autonomic modulation.
Conclusion: This is the first study that has evaluated the impact autonomic modulation has on electrophysiological properties in humans. The study has shown that CV dynamics is impacted by scar and influenced by autonomic modulation which enhanced CV heterogeneity and distribution of pivot points. This study has shown that autonomic modulation enhances an environment that increases susceptibility to re-entry formation. This study provides further insight to the impact of autonomic remodelling in AF. ❑
Figure 1