Highlights
-
The autonomic nervous system plays a critical role in the aetiopathogenesis of atrial fibrillation (AF).
-
Neuromodulation of the autonomic nervous system has been a topic of increased interest in the management of AF.
-
Ablation of ganglionated plexi, ethanol ablation of the vein of Marshall, renal denervation and pulmonary vein isolation have demonstrated promising results in well-selected patients with AF.
-
Transcutaneous vagus nerve stimulation might be a non-interventional treatment option to increase AF-free survival.
-
There are still unanswered questions regarding which patients would benefit the most from neuromodulation strategies.
Atrial fibrillation (AF) is the most common cardiac arrhythmia and is associated with significant morbidity and healthcare utilization.1–3123 In attempts to blunt the effect of this common and complex entity, there has been an increasing interest in improving our understanding of the pathogenic basis governing AF.4–13456789...13 Although pulmonary vein isolation (PVI) remains the gold standard, adjunctive ablative approaches for patients with persistent forms of AF have been studied but appear to yield modest additional benefits at most when empirically applied.8,14–2781415161718...27 Furthermore, the most recent technological advancements have focused more on improving procedural efficiency through novel technologies and improving patient selection.26,28–38262829303132...38 One area of heightened research is unravelling the relationship between the cardiac autonomic nervous system (ANS) and the establishment and maintenance of AF, thereby developing neuromodulatory interventions.26,39–47263940414243...47 In this article, we dive into the link between the ANS and AF and discuss the established and emerging strategies to manage AF via neuromodulation.
The neurocardiac axis
Understanding the neurocardiac axis and how it affects AF pathogenesis is essential to comprehending the role of neuromodulation in the management of AF.
The link between the heart and the brain has long been established. In the late 1800s, Gaskell and Langley were the first to describe the basic structure of the ANS to add clarity to how it regulates the cardiovascular system.48Expand Reference Nearly four decades later, Cannon described homeostasis, the process whereby the ANS regulates critical physiological parameters.49Expand Reference In subsequent years, many observational studies have focused on patients with primary nervous system pathologies and how these disorders affect the regulation of the cardiovascular system.50Expand Reference
The modulation of the neurocardiac axis in the setting of systemic illnesses, such as embolic stroke and AF, or subarachnoid haemorrhage and stress-induced cardiomyopathy has only recently been better understood and has led to new therapeutic interventions. Based on the data from many clinical studies, there are multiple cortical and subcortical regions in the central nervous system (CNS) that form larger networks that innervate the cardiovascular system.51,525152 These networks include brainstem regions, such as the ventrolateral medulla, and areas in the cortex, such as the dorsal cingulate cortex. However, the area that plays the greatest role in the regulation of the heart–brain axis is the insular cortex.51,525152 The posterior insular cortex receives the cardiac input via the thalamus.53–55535455 Heart rate increases and decreases with the stimulation of the rostral and caudal insular cortices, respectively.56Expand Reference Moreover, the stimulation of the right and left insula leads to increased sympathetic and parasympathetic tones, respectively.57Expand Reference
The sympathetic nervous supply to the heart originates from neurons located in the intermediolateral column of the upper thoracic spinal cord.58Expand Reference These neurons form synapses in the cervicothoracic stellate ganglia. The sympathetic effects of these neurons are mediated by B1 receptors on cardiac myocytes. These receptors send their signals via a G-protein-coupled mechanism that increases the levels of cyclic adenosine monophosphates.59,605960 The parasympathetic fibre supply to the heart originates from the dorsal motor nucleus in the medulla, travels along the vagus nerve and finally forms synapses with postganglionic neurons in the intrinsic (autonomic) cardiac ganglia. Acetylcholine is the main neurotransmitter for these parasympathetic fibres. Acetylcholine binds to M2 muscarinic receptors after being released by postganglionic parasympathetic fibres, leading to the opening of potassium channels and ultimately a decrease in heart rate and contractility. In addition, there is cerebral lateralization in cardiac autonomic control, with the right cerebral hemisphere predominantly modulating the sympathetic activity.61Expand Reference
There are other neuromodulators released from the myocardium and coronary vessels that regulate the sympathetic and parasympathetic tones. Angiotensin II released by the myocardium increases the sympathetic activity, while C-type natriuretic peptide increases the parasympathetic activity.62Expand Reference
Electrocardiographic changes are frequently observed after brain injuries. Sympathetic hyperactivity after brain injuries leads to an enhanced calcium influx, which alters the endocardial conduction system.63,646364 These changes are more common in the first 24 h after acute neurological injuries. Furthermore, pathophysiological activation of the insular cortex by stroke or epileptic seizure or under conditions of severe emotional stress could predispose to electrocardiogram changes, cardiac arrhythmias and sudden death via autonomic effects.65Expand Reference
When the regulation of the ANS is impaired, distinct cardiovascular changes occur. Autonomic dysregulation can lead to either increased or decreased sympathetic tone. Primary CNS pathologies that are associated with autonomic dysfunction include cerebrovascular diseases (strokes) spinal cord diseases and neurodegenerative diseases (Parkinson’s disease).66,676667 Peripheral nervous system pathologies that lead to ANS failure include neuropathies secondary to diabetes mellitus, paraneoplastic syndromes and autoimmune conditions.66Expand Reference
The clinical features of autonomic dysfunction include presyncope, syncope and loss of balance. Presyncope and syncope in the setting of autonomic dysfunction are usually due to orthostatic hypotension.67Expand Reference This can usually be treated with conservative measures, such as maintaining adequate hydration, increasing sodium intake and avoiding strenuous activity after meals or, in select cases, via cardioneural ablations.68–7168697071
Cardiovascular manifestations are observed in many neurological disease states in addition to autonomic dysfunction. Both ischaemic and haemorrhagic strokes are associated with bradycardia, heart block and tachyarrhythmias. AF is the most frequently observed arrhythmia after a stroke.72,737273 These cardiac manifestations post-stroke are associated with an increased mortality.74Expand Reference
Another example highlighting the brain–heart connection is cardiac arrhythmias observed in temporal epilepsy. Ictal and post-ictal heart blocks can be observed in this case. Although these rhythms are frequently benign, they can convert into life-threatening arrhythmias, such as ventricular fibrillation, ventricular tachycardia and supraventricular tachycardia.75–7875767778
A better understanding of the anatomic basis of the neurocardiac axis and the alterations that occur in various pathological states has led to the development of new therapies aimed at modulating the adverse effects encountered during disease states. For example, many refractory tachyarrhythmias can be treated by nerve blocks of the cardiac sympathetic ganglia. Vagal nerve stimulation has also been shown to protect against both atrial and ventricular arrhythmias.79Expand Reference
Our understanding of the heart–brain axis and its clinical implications has expanded considerably over the last half-century. However, there is still more progress needed in this important area. The pathophysiology of the cardiovascular effects of nervous system pathologies, such as subarachnoid haemorrhage or traumatic brain injury, is not well understood. Further research that uses expertise across multiple specialities including cardiology, neurology and critical care, will improve our understanding of the neurocardiac axis and lead to new innovative treatments for patients.
Key intrinsic cardiac neuroanatomy
Central to the discussion on the ANS and cardiac function is the understanding of the difference between the distribution of sympathetic and parasympathetic nerve fibres and their ganglia. In the sympathetic nervous system, the pre-ganglionated fibres are short, with the ganglia residing in the spinal cord, while the post-ganglionated fibres are long and terminate in the effector organ (e.g. the heart). This contrasts with the parasympathetic system, in which the pre-ganglionated fibres are long, with the autonomic ganglia residing within the target organs.80Expand Reference In the heart, this takes the form of clusters of ganglia, distributed within the epicardial tissue around the atria and the ventricle and called ganglionated plexus (GP).81Expand Reference While the precise locations, densities and distributions of these GPs may vary, Armour et al. described their typical locations and set forth the nomenclature.82Expand Reference
The following anatomical areas contain most of the intrinsic cardiac ganglia: superior (anterior) right atrial GP on the posterosuperior surface of the right atrium (RA) in the region of the superior vena cava–RA junction, inferior (posterior) right atrial GP in the region of interatrial groove, superior left atrial GP on the posterosuperior surface of the left atrium (LA), inferior (posterolateral) left atrial GP on the posterolateral surface of the LA and posteromedial left atrial GP on the posteromedial medial surface of the LA near coronary sinus ostium. The vein of Marshall (VOM) may also be considered a part of the cardiac ANS because parasympathetic fibres from the VOM innervate the surrounding left atrial structures and the coronary sinus (the Marshal tract GP).83Expand Reference This is further illustrated in Figure 1.
Figure 1: The schematic view of ganglionated plexi
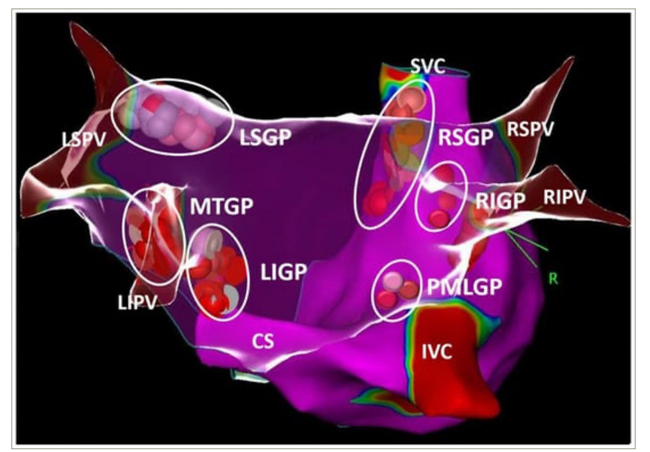
White, pink and red dots show the distribution of ablation points based on fragmented bipolar electrograms Reproduced with permission from Aksu et al.83Expand Reference
CS = coronary sinus; IVC = inferior vena cava; LIGP = left inferior atrial ganglionated plexi; LIPV = left inferior pulmonary vein; LSGP = left superior atrial ganglionated plexi; LSPV = left superior pulmonary vein; MTGP = Marshall tract ganglionated plexi; PMLGP = posteromedial left atrial ganglionated plexi; R = right; RIGP = inferior right atrial ganglionated plexi, RIPV = right inferior pulmonary vein; RSGP = right superior atrial ganglionated plexi; RSPV = right superior pulmonary vein; SVC = superior vena cava.
Autonomic nervous system and atrial fibrillation
The relationship between AF and alterations of ANS has been established.84,858485 Coumel et al. hypothesized that changes in vagal tone act as a trigger for AF.86Expand Reference This has been complemented by early observations of a circadian variation in the AF burden in some individuals, which was hypothesized to be likely linked to changes in autonomic tone throughout the day.87,888788 Other signs of ANS involvement in AF are the phenotype of vagal AF, in which the patients are observed to have AF triggered during the periods of bradycardia. These associations have been elucidated by the findings of multiple basic science and clinical studies.64,89,90648990
Inputs from the ANS have been implicated in the pathogenesis of AF via multiple proposed mechanisms although many questions remain unanswered.4Expand Reference These include alterations in the action potential duration, induction of rapid firing of early afterdepolarization and changes in atrial refractoriness induced by modifications in parasympathetic tone.91,929192 Furthermore, it has been noted that the alteration of autonomic tone via the injection of agents with parasympathetic properties into epicardial fat pads rich in GPs can result in increased inducibility and maintenance of AF.93Expand Reference Additionally, in vitro canine studies have shown that autonomic nerve stimulation can lead to pulmonary vein firing and induce AF.94Expand Reference
Neuromodulation for the control of atrial fibrillation
Catheter-based therapies with PVI have been the backbone of AF management and are currently recommended by most major societies.3,95,9639596 However, given the need for additional strategies to improve outcomes in AF, several catheter-based techniques to modulate the ANS have been explored. These include intrinsic neuromodulation within the heart and anatomic nervous inputs originating outside the heart (Table 1).
Table 1: Techniques for neuromodulation in atrial fibrillation
|
Location |
Neuromodulation technique |
|
Cardiac targets |
Endocardial ganglionated plexus ablation |
|
Vein of Marshall ethanol infusion |
|
|
Extracardiac targets |
Renal denervation |
|
Stellate ganglion blockade |
|
|
Baroreflex therapy |
|
|
Transcutaneous vagal nerve stimulation |
Endocardial neuromodulation
One technique gaining traction in the management of AF is neuromodulation via endocardial-targeting GPs. Cardioneuroablation is based on the observation that the heart rate is higher after PVI due to the destruction of GPs. GP might be identified during an electrophysiological study (EPS) via an electrogram analysis. These include the identification of fractionated atria electrograms with one of the following characteristics as outlined in Table 2 and illustrated in Figure 2. Once identified, it can be targeted for radiofrequency ablation using catheter-based techniques.97–100979899100 However, a fragmented electrogram-guided strategy has some limitations in both specificity and sensitivity. Specificity may be limited, as fragmented electrograms may represent the areas of atrial fibrosis, pulmonary vein (PV) potentials or double potentials. Moreover, the adipose tissue surrounding the heart can infiltrate the atrial myocardium, causing heterogeneous activation and resulting in the presence of fragmented electrograms.
Table 2: Characteristics of electrogram to determine the location of ganglionated plexi
|
EGM characteristics |
Criteria |
|
Normal EGM |
<4 deflections or ≤40 ms duration |
|
Low-amplitude fractionated EGM |
≥4 deflections or >0.7 mV amplitude |
|
High-amplitude fractionated EGM |
≥4 deflections, >0.7 mV amplitude or >40 s duration |
EGM = electrogram; ms = millisecond; mV = millivolt; s = second.
Figure 2: Three types of bipolar atrial electrogram for ganglionated plexus mapping
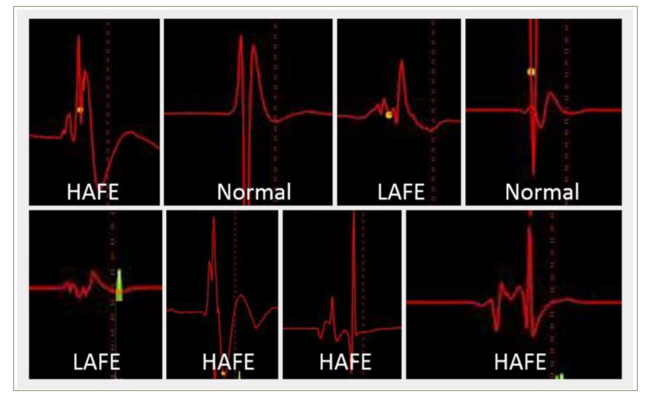
Reproduced with permission from Aksu et al.83Expand Reference
HAFE = high-amplitude fragmented electrogram; LAFE = low-amplitude fragmented electrogram; Normal = normal atrial electrogram.
The identification of GPs through an EPS may be achieved via high-frequency simulation (HFS), in which case the LA is paced with the ablation catheter at a rate that exceeds the intrinsic sinus rate. Additionally, HFS is applied at -20 Hz, between 10 and 140 V and at a 1–10 ms pulse width in the anatomical regions where GPs are known to present.101Expand Reference
Ablation of these GPs in these locations has been associated with decreased rates of AF when compared with PVI alone, although ablation techniques and acute procedural endpoints tend to vary amongst centres.26,10226102 Long-term effects of GP ablation performed adjunctive to PVI remain unknown, such as the durability due to the phenomenon of nerve regeneration and reinnervation following RF and cryoablation. As non-thermal technologies for PVI, such as pulsed-field ablation, proliferate in clinical practice, the necessity for deliberate GP ablation may increase as the delivered pulsed electric fields tend to spare GPs when endocardial ablation is performed.41,103,10441103104
Ethanol ablation of the vein of Marshall
There is increasing evidence that intrinsic cardiac innervation via the ANS, which plays a significant role in the pathogenesis of AF, is located in the LA. The ligament of Marshall and its extension as the VOM have been considered parasympathetic and sympathetic inputs and implicated in the pathogenesis of AF by multiple proposed mechanisms.105,106105106
To examine the parasympathetic denervation via ethanol infusion in the VOM (which connects to the ligament of Marshall), Báez-Escudero et al. performed a retrograde ethanol injection into the VOM at the time of AF ablation. Of the 133 patients enrolled in the study, successful VOM ablation was performed in 80 patients, with acute elimination of parasympathetic responses and AF inducibility.107Expand Reference
From a clinical standpoint, ethanol infusion in the VOM has been shown to have some benefits over catheter ablation alone, as was demonstrated in the Vein of Marshall Ethanol Infusion for Persistent AF (VENUS) trial (ClinicalTrials.gov identifier: NCT01898221) and other studies.8,1088108 In this randomized single-blinded trial, which included 350 patients with persistent AF, participants were randomized either to catheter ablation alone or to catheter ablation with the addition of ethanol infusion in the VOM. This finding of improved recovery from AF was substantiated by a meta-analysis focused on the long-term outcomes when VOM ethanol infusion was added to PVI compared with PVI alone.109Expand Reference Regarding safety, a study of 700 patients by Kamakura et al. showed that VOM ethanol infusion was feasible with relatively low complication rates, and the main complication was delayed tamponade at a rate of 0.8%.110Expand Reference Additional research on this subject continues, with favourable findings suggesting that apart from the addition of VOM ethanol infusion to PVI, AF recurrence could also be decreased further if linear lesion sets are added (dome, mitral and cavotricuspid isthmus).111Expand Reference
Sympathetic denervation
Given the association of AF with heightened sympathetic activity, sympathetic denervation has been evaluated extensively. One explored strategy is renal sympathetic denervation, which is a technique that has been targeted originally for the control of hypertension. However, the results of renal denervation, as demonstrated via the SYMPLICITY HTN-3 trial (Renal Denervation in Patients With Uncontrolled Hypertension; ClinicalTrials.gov identifier: NCT01418261), failed to show blood pressure reductions with renal artery denervation.112Expand Reference
In this approach, ablation at the bifurcation of the renal artery was performed. In the Atrial Fibrillation Reduction by Renal Sympathetic Denervation trial by Feyz et al., following renal artery denervation, the AF burden decreased from 1.39 min/day prior to renal denervation to 0.94 min/day 12 months post-renal denervation (p=0.03).113Expand Reference This was associated with statistically significant improvements in quality of life.
To substantiate these findings further, the Effect of Renal Denervation and Catheter Ablation versus Ablation alone on Atrial fibrillation Recurrence Among Patients with Paroxysmal Atrial Fibrillation and Hypertension (ERADICATE-AF; ClinicalTrials.gov identifier: NCT01873352) trial was conducted.114Expand Reference In this trial involving 302 patients, patients were randomized either to PVI alone or to PVI + renal denervation. During the 12 months of follow-up post-procedure, freedom from AF, atrial flutter or atrial tachycardia was observed in 72.1% of patients undergoing renal denervation in addition to PVI, compared with only 56.5% of patients undergoing PVI alone. Interestingly, significant reductions in systolic blood pressure were observed in persons undergoing renal denervation, a finding that was not observed in the Simplicity HTN-3 trial.112Expand Reference Furthermore, renal denervation has been shown in pilot studies to be a means to prevent subclinical AF in patients with a history of hypertension or heart disease at risk for the development of AF.115Expand Reference More recently, however, in the long-term follow-up for the AFFORD trial, there was no significant reduction in the AF burden during the 3-year follow-up. In patients with both AF and hypertension, treatment of hypertension should aim for current blood pressure guidelines to reduce stroke, bleeding and other adverse outcomes.116Expand Reference Considering there were fewer atrial arrhythmia recurrences and better blood pressure control among participants treated with renal denervation and PVI in the ERADICATE-AF trial, renal denervation in addition to PVI might be reasonable in well-selected patients with AF and uncontrolled hypertension.
Stellate ganglion block
Sympathectomy via surgical approaches and percutaneous stellate ganglion block has gained popularity in the management of ventricular tachycardia storm.117–123117118119120121122123 This has been shown to have acute benefits in reducing AF inducibility in animal studies and small human trials.124–126124125126 More recently, the use of stellate ganglion block at the time of coronary artery bypass graft was studied in a small randomized controlled trial of 40 patients.124Expand Reference Participants were randomized either to a control group or to an ultrasound-guided left stellate ganglion block group with the injection of 10 mL of 2% lidocaine. While statistically significant decreases in arrhythmia were observed in the intraoperative period, no difference between the two groups was observed during the early postoperative period.127Expand Reference
Additional neuromodulatory techniques for atrial fibrillation management
While not catheter-based therapies, a few approaches that result in neuromodulation have been examined to augment the standard management of AF, one of which includes transcutaneous vagus nerve stimulation.
The principle governing transcutaneous vagus nerve stimulation is that low levels of stimulation of the vagus nerve can decrease parasympathetic tone and blunt vagally mediated AF.128,129128129 Beyond alterations in autonomic tone, low-level transcutaneous vagus nerve stimulation (LLTS) has been associated with decreased inflammatory cytokines, which may function as a driver for AF.128Expand Reference In the Transcutaneous Electrical vAgus nerve sTimulation to suppression Atrial Fibrillation (TREAT AF; ClinicalTrials.gov identifier: NCT02548754) trial, patients were randomized to the LLTS group (20 Hz and 1 mA), in which it was administered via an ear clip (tragus), versus sham. After a 6-month follow-up period, the AF burden was 85% lower in the treatment group versus the sham group (0.15, 95% confidence interval 0.03–0.65, p=0.011).130Expand Reference In a recently published study, the effect of acupuncture at the auricular branch of the vagus nerve on the autonomic system was investigated in humans. In comparison with placebo acupuncture, acupuncture at the auricular branch of the vagus nerve caused a significant reduction in heart rate and an increase in overall heart rate variability parameters in favour of vagal tone.131Expand Reference Further investigation on this subject may be worthwhile; however, the potential loss of effect with the cessation of the device use was an unattractive feature. Nonetheless, its role as an adjunct tool for AF management in select patients might be considered if its utility is validated in large studies.
Summary position
According to the published literature, endocardial ablation of GPs in conjunction with PVI confers a higher success rate than the PVI-alone strategy when treating patients with paroxysmal AF.102,132102132 On the other hand, endocardial ablation of GPs without PVI or surgical GP ablation has no role in clinical success.102,133102133 Although the published data to date do not yet support endocardial GP ablation as an alternative to PVI, in appropriately selected subgroups of patients, endocardial GP ablation in addition to PVI might be a potential alternative to PVI-alone strategy.
VOM ethanol ablation in addition to PVI may play an important role in preventing AF recurrence in patients with persistent AF and might be used as a first-line strategy in patients with persistent AF. Adding renal denervation to PVI should only be an option in patients with AF with accompanying uncontrolled hypertension. Larger randomized controlled studies are needed to define suitable candidates for these alternative strategies.
Conclusion
As our quest for improved outcomes in AF continues, developing management strategies to tackle the disease process on its multiple pathophysiological fronts is warranted. While not universally applicable, neuromodulation as an additive strategy in appropriately selected patient populations may be useful.
















