The first-in-human implantation of a leadless pacing system occurred already more than 10 years ago.1 The first-generation ventricular leadless pacemaker could provide only asynchronous ventricular pacing (ventricular, ventricular, inhibited [VVI], or ventricular, ventricular, inhibited, rate response [VVIR]), limiting its indications to patients with atrial fibrillation and severe bradycardia, those precluded for implantation of a transvenous pacemaker or those with an expected low ventricular pacing burden.2,3
In 2020, the first leadless AV pacing device called Micra AV (model MC1AVR1, Medtronic, Inc., Minneapolis, MN, USA) was approved by the US Food and Drug Administration (FDA) for clinical use. This device had exactly the same design as the Micra VR but contained a new algorithm enabling a ventricular, dual, dual (VDD) pacing mode in which the accelerometer is used to detect the atrial contraction and ensure AV synchronization (Figure 1A). This new algorithm resulted in an average atrio-ventricular synchrony (AVS) of 89.2% in patients with complete AV block and normal sinus rhythm at rest in the MARVEL 2 (Micra Atrial tRacking using a Ventricular accELerometer 2; ClinicalTrials.gov identifier: NCT03752151) study.4
Figure 1: Atrial sensing by the Micra AV device: How does it work?
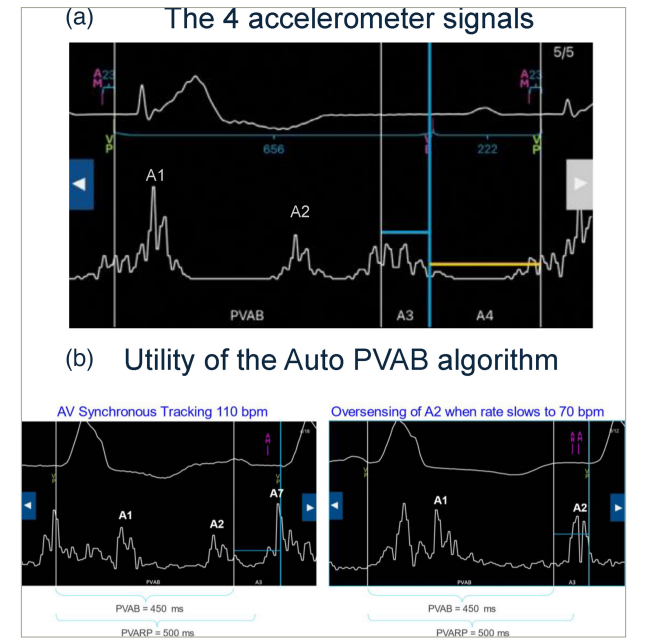
(A) The four distinct accelerometer signals. As a reminder, A1 occurs at the beginning of ventricular systole and represents the closing of the mitral and tricuspid valves. A2 occurs at the completion of ventricular systole and represents the closing of the aortic and pulmonary valves. A3 occurs during ventricular diastole and represents the passive filling of blood from the atrium to the ventricle. A4 signal occurs when the atrium contracts and pushes blood into the ventricle. (B) First-generation Micra AV has a fixed PVAB, which limits the highest achievable upper tracking rate. In this example, in a Micra AV patient, the A2 signal is appropriately blanked at 110 bpm by a 450 ms PVAB. However, the A2 signal timing occurs later, as the heart rate slows to 70 bpm, meaning that the same PVAB setting does not appropriately blank the A2 signal and the A2 signal is oversensed in the A3 window. To prevent this inappropriate oversensing, Auto PVAB was included in Micra AV2 to automatically switch to a shorter PVAB at higher rates.
AV = atrio-ventricular; bpm = beats per minute; PVAB = post-ventricular blanking; PVARP = post-ventricular atrial refractory period.
Nevertheless, following studies evaluating AVS in real life reported less attractive data with a highly variable ambulatory AVS from 33 to 91%, depending on the pacing indication, underlying heart rate and ventricular pacing percentage.5–8 Achieving a high AVS is particularly challenging when the sinus rate is higher than 80 beats per minute (bpm). In the AccelAV (Accelerometer Sensing for Micra AV; ClinicalTrials.gov identifier: NCT04245345) study, manual programming improvements increased the average AVS from 71.9 to 82.6% in patients with continuous ventricular pacing.7 The average AVS improvement observed was more prominent for higher sinus rates (90–100 bpm; 70.9–88.4%, p<0.001) compared with lower sinus rates (60–70 bpm; 78.6–83.7%, p=0.068).7 In 2023, Wu et al. published a large meta-analysis reporting an average AVS of 78.9% and a significant increase of 11.3% AVS, thanks to programming optimization.9 Recently, the 12-month results of the worldwide Micra AV post-approval registry (an ongoing prospective single-arm observational registry in a real-world setting at 3 years post-implant) confirmed the safety and effectiveness of the device.10 The complication and revision rates remain low: 3.7 and 1.5%, respectively, at 1 year. The reported median AV synchrony index, a device parameter considered a surrogate for the true AVS, was 79.4% in patients with >90% ventricular pacing, without any specific programming recommendations.10
To overcome the initial limitations of Micra AV, an algorithm upgrade was designed to increase AV-synchronous efficiency while reducing the manual programming burden. This algorithm update was implemented in a new generation of Micra AV called Micra AV2 (model MC2AVR1, Medtronic, Inc., Minneapolis, MN, USA), which is now available for clinical use. The different proposed improvements by this new device are further described in this article.
First, it was attempted to improve AVS at higher sinus rates by adaptations of atrial sensing parameters, including the A3 threshold auto-adjusting algorithm. In the original Micra AV, high rates would sometimes cause this algorithm to adjust the A3 threshold too high, resulting in undersensing of the atrial mechanical contraction. It was even proposed to disable the original Auto A3 threshold feature and adjust a fixed A3 threshold based on manual measurement of the A3/A4 signal amplitudes to maintain AV synchrony at higher sinus rates.11 Micra AV2 has a new algorithm called Auto+ A3 threshold to automatically adjust the A3 threshold. Auto+ A3 uses filtered, true A3 signal amplitudes to automatically set the A3 threshold above the A3 signal but below the A7 signal (A7=summated A3+A4 signal). Sensing of the A7 signal will allow tracking at higher sinus rates (>85 bpm) (Medtronic, unpublished data).
Second, the AV conduction mode switch was enhanced to allow minimizing ventricular pacing in patients with paroxysmal block. This is an important feature of Micra AV to limit the amount of right ventricular pacing and to maximize device longevity in patients with paroxysmal AV block or periods with intrinsic ventricular rhythm by switching to a mode without atrial sensing.11 In Micra AV, this feature leads to switching from VDD to VVI pacing at a fixed lower rate of 40 bpm. This algorithm has the risk of provoking low true AV synchrony in patients experiencing periods of intrinsic ventricular rhythm ≥40 bpm during AV block (e.g. sinus rate >80 bpm and 2:1 block).12 In the new Micra AV2 device, the lower rate for this mode is programmable between 40 and 70 bpm (nominal low rate of 50 bpm), allowing for more programming flexibility while enhancing the true AV synchrony and reducing the risk of developing symptoms related to low AVS (Medtronic, unpublished data).
Third, the maximal upper tracking rate can be programmed to allow tracking up to 135 bpm compared with 115 bpm in the Micra AV, thanks to a new Auto post-ventricular atrial blanking (PVAB) algorithm permitting the reduction of PVAB to a minimal value of 425 ms when the heart rate increases (Figure 1B) (Medtronic, unpublished data). This new algorithm is probably the most challenging of the new Micra AV2 device, as it requires manual setup and correct interpretation of the four accelerometer signals by the clinician (Figure 1A). The Auto PVAB algorithm automatically switches between two PVAB settings, minimum (Min) PVAB and maximum (Max) PVAB, depending on the patient’s heart rate. In addition, the interval between the A1 and A2 signals shortens as the rate increases (analogous to QT interval shortening), and the Min PVAB is used for blanking at rates above the PVAB switch rate, nominally 90 bpm (Medtronic, unpublished data). To avoid signal oversensing and ensure patient safety, an assessment should be performed to raise the patient’s heart rate to the PVAB switch rate (either via temporary pacing or exercise). This will allow for the determination of the lowest Min PVAB setting that sufficiently blanks the A2 signal. A 425 ms Min PVAB setting will allow for tracking up to 135 bpm; however, it is important to note that this may not be appropriate for all patients due to individual differences in A2 signal timing.
Fourth, the automatic atrial sensing setup was updated, reducing the need for manual programming by >50% at the first device control post-implant (Medtronic, unpublished data). The original feature was designed to automatically set up atrial mechanical sensing parameters, including the atrial sensing vector, A3 and A4 threshold and A3 window end-related parameters after the implant. The device collects A3 and A4 signal data in ventricular pacing with inhibition and dual chamber sensing (VDI) mode and then refines settings in VDI and VDD modes. In Micra AV2, new filtering of the A3 and A4 signal data has been incorporated for a more accurate method of setting the atrial mechanical sensing parameters.
All these algorithms or device improvements were made to improve patients’ conditions and AVS, but further studies are needed to prove their efficacy in real-life AV synchrony and patient symptoms.
In addition to these algorithm improvements, the Micra AV2 has a projected median longevity of 15.6 years compared with the median projected longevity of 10.8 years for the Micra AV device (Medtronic, unpublished data).
Besides Micra AV2, other leadless atrio-ventricular pacing systems are arising. The AVEIR DR (Abbott, Abbott Park, IL, USA), which received FDA approval in July 2023 and CE marking in June 2024, permits leadless dual-chamber pacing with atrial pacing and a higher reported AV synchrony at 6 months of follow-up.13 The AVEIR DR system offers for the first time a leadless ‘physiological’ solution for patients suffering from sinus node dysfunction, while the Micra AV2 remains an option for patients with high-degree AV block with normal sinus function. The AVEIR DR requires the implantation of two separate devices, and long-term data on its performance (battery longevity, AV synchrony and implant-to-implant communication) will also be necessary for widespread clinical adoption. Future clinical studies will need to clarify the exact role of each device in real-world settings.
In conclusion, these innovations emphasize that leadless pacemakers are considered an alternative to conventional ventricular and dual-chamber pacemakers. The technology is evolving very rapidly, and leadless AV-synchronous conduction system pacing could represent an exciting and logical future evolution.












