The Ground-BrEAking Electroporation-based inTervention for Atrial Fibrillation (BEAT-AF) treatment project is an initiative funded by the European Commission from the European Union’s Horizon 2020 research and innovation programme, and managed under the European Health and Digital Executive Agency (grant number 945125).1,212 The study was also partly funded by IHU LIRYC ANR-10-IAHU-04. This pioneering 5-year programme commenced on 1 March 2021, and is spearheaded by Professor Pierre Jaïs from Bordeaux University, Bordeaux, France.
Atrial fibrillation (AF) is the most common cardiac arrhythmia and a major contributor to cardiovascular healthcare costs, affecting over 10 million individuals across Europe.3Expand Reference AF not only severely impacts the quality of life but also increases the risk of stroke, heart failure, dementia and mortality.4Expand Reference Catheter ablation, targeting the isolation of pulmonary veins and the prevention of arrhythmia recurrences, is the primary treatment approach using either radiofrequency (RF) or cryothermal energy.5Expand Reference However, these traditional methods are not without their drawbacks, including the indiscriminate ablation of all tissue types, technical demands, prolonged procedure times and a propensity for severe complications due to their thermal nature.6Expand Reference
In response to this need and to reduce the substantial burden of AF, the BEAT-AF trial (Ground-Breaking Electroporation-based Intervention for PAROXysmal Atrial Fibrillation Treatment [BEAT PAROX-AF]; ClinicalTrials.gov identifier: NCT05159492) was designed as an ambitious, multicentre randomized study to explore if pulmonary vein isolation using pulsed electric field (PEF) catheter ablation, a non-thermal approach, could be more effective and safer than the gold-standard RF and cryoablation methods.7Expand Reference To actualize this, Professor Jaïs and his team have used their secured support to facilitate the collaboration between nine clinical centres across Europe, resulting in a consortium that includes nine partners from France, Germany, Belgium, Austria and Czechia.
In this interview, we speak with Professor Jaïs to explore this pioneering trial, who has been driven by decades of experience in cardiac arrhythmia treatments and motivated by the limitations of traditional ablation methods. The discussion delves into the operational aspects of the trial, including the design, collaborative nature and potential impacts on clinical practice and patient care. The interview also addresses the broader implications for healthcare systems, emphasizing the need for more effective and efficient treatment modalities in cardiology.
Q: What prompted the initiation of the Ground-BrEAking Electroporation-based inTervention for Atrial Fibrillation trial, and how does it aim to address the current challenges in treating atrial fibrillation?
I have been performing catheter ablation for cardiac arrhythmias for around 30 years. In the 1990s, at the University Hospital, Bordeaux, we actually pioneered AF ablation using RF energy with focal catheters.
Now, a focal catheter is quite small, typically around 2.4 mm in diameter, and is designed to create small lesions about 5 mm in diameter and approximately 3–4 mm in depth. In the 1900s, we recognized the necessity of navigating around the pulmonary veins to ensure that the small lesions created were hopefully coalescent and adjacent so that there were no gaps in the encircling lesion. However, it was clear that this process was not optimal, as we were using catheters originally designed for treating focal targets.
Before the advent of AF ablation, every ablation procedure targeted specific, discrete focal points, such as those used in treating atrial tachycardia, accessory pathway-mediated tachycardias or atrioventricular nodal reentrant tachycardia. However, ablation for AF introduced a completely different challenge. This procedure requires the creation of linear or circumferential lesions around the pulmonary veins – a task that is challenging to achieve with small point lesions designed for focal targets. As a result of this, I have always been fascinated by the possible improvements that could be made to this procedure to translate into better care for our patients.
Therefore, around 8 years ago, I was contacted by a start-up company from the USA who were exploring the possibility of using electroporation as a new technique for treating cardiac arrhythmias.
The concept of electroporation itself was not new, as it has been used in oncology for many decades. However, the technological capability to apply it to cardiac tissue was novel. The dedication of the companies and individuals involved in progressing this has been remarkable, and I have been incredibly honoured and excited to have been able to collaborate with them in developing electroporation as a viable technique for cardiac applications.
From first-hand experience using this technology, I can say that one major advantage is that it simplifies and speeds up the process of AF ablation. However, I would not go as far as to say it makes the procedure easy – that would not be correct.
From my perspective, the advancement is truly significant because it enables us to treat more patients efficiently within the same timeframe, potentially allowing us to treat twice the number of patients we treat now. Moreover, as the process is less complex, the learning curve and training period are considerably shorter. This is also crucial, as we need to improve upon the current access rate, where only an extremely modest 3% of patients can access catheter ablation. Therefore, if we can make it faster and simpler, my hope is that we can help more patients, and this is what really motivated me to initiate the BEAT-AF trial.
I would like to say that I have had two key pinnacles in my career. The first was when we first understood that AF was triggered by the pulmonary veins, which led us to consider strategies around how to ablate and reduce the morbidity and mortality in our patients, and the second was this novel non-thermal technique, which uses PEF energy for ablation. PEF energy, which is being trialled in BEAT-AF works, uses extremely short high-voltage pulses to create nanoscale pores in cell membranes, which we hope is much safer for our patients.
Video demonstration
For a visual demonstration of how the RF and PEF catheters behave on their way to the pulmonary vein, please refer to the accompanying video (accessible at: https://www.youtube.com/watch?v=IE_acuC5DeI), which showcases multiple side-by-side simultaneous camera shots. Copyright: This content was filmed and funded by the BEAT–AF. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement number 945125. This content is reproduced with permission from the BEAT-AF, 2024, www.beat-af.com.
Q: Could you elaborate on how pulsed electric field energy offers a promising alternative for achieving durable pulmonary vein isolation in terms of safety?
Therefore, this technique is a completely different way of treating the areas responsible for arrhythmia. With cryoablation or RF ablation, you create a thermal lesion, and there is a thermal injury associated with the diffusion of extreme cooling or an increase in temperature in the cardiac wall. In addition, you have absolutely no control over how deeply this effect will propagate. If there is too much of a temperature change, extracardiac damage is possible, and if the temperature change is not large enough and does not reach transmurality, you risk possible recurrences. Hence, with these energy sources, it is impossible to adjust the amount of energy to ensure that it includes but stops right at the end of the cardiac wall.
Now, there have been several developments that have allowed us to start adjusting this to achieve the right dose; however, this is never perfect; in fact, it is far from that. It is also the reason we observe some of those complications, such as phrenic nerve palsy. Phrenic nerve palsy is quite common with cryoablation because the phrenic nerve is squeezed between the balloon and extracardiac tissues. It also explains atrial–oesophageal fistulas, a rare but serious complication; they may happen with cryoablation and even more so with RF. And then, there are the not-so-obvious complications. For example, these thermal energies can create retractile scars, particularly if you are using RF ablation.
A retractile scar is definitely something you do not want to have. If the catheter is positioned within the veins during the procedure, it can cause permanent stenosis. It can also lead to stiff left atrial syndrome, an underrecognized condition. For this to occur, you probably need to have received a lot of ablations, so it is more likely in patients with persistent AF, but if it does occur, it can be a major issue.
If the atrium can no longer fully expand, it loses its crucial reservoir and conduit functions, which are essential for maintaining proper cardiac physiology and ensuring adequate filling of the left ventricle. This impairment can lead to symptoms of heart failure, which are particularly challenging to manage because there are no drugs specifically tailored to address this issue. Although it is not a common problem, some literature reports its occurrence at rates as high as 5–7% indicating that it is not a trivial concern either.
Now where this technique differs is that electroporation is non-thermal.
However, one challenging aspect we must acknowledge with electroporation is that, in contrast, when discussing RF ablation, virtually every device supplier offers a similar solution – it is the same recipe. Typically, this involves using 500 kHz, which generates resistive heat in the first few millimetres of tissue, and then, the heat wave propagates from there. With electroporation, we are using electricity as well but in a completely different manner. This manner can lead to the irreversible rupture of the cell membrane, but what I find fascinating is that there is some tissue selectivity. However, this is actually extremely fortunate because the cardiac cells we want to remove are extremely sensitive to this energy, much more so than the phrenic nerve or the oesophagus that are adjacent. This offers a significant advantage because even if the catheter generates an electric field exposing all the cells within that field, only those cells sensitive to the energy level used will be affected. For example, if the oesophagus falls within this electric field, its cells, due to their different nature, will not be impacted.
However, as I mentioned earlier, this may depend on the specific electroporation protocol used. Unlike RF ablation, where the approach is more standardized, there are countless combinations available to create what we might call a waveform in electroporation. These waveforms can vary significantly from one vendor to the next, offering a diverse range of treatment options. As a result of this, it is possible that in the future, we may see some thermal effects and some thermal complications with electroporation. It is not completely impossible. However, with the current protocols I have worked with – namely, Boston Scientific’s FARAPULSE™ pulse-field ablation (PFA) System (Boston Scientific, Marlborough, MA, USA), Medtronic’s Affera™ Mapping and Ablation System (Medtronic plc, Dublin, Ireland) and Biosense Webster’s VARIPULSE™ PFA Platform (Biosense Webster, Irvine, CA, USA) – these three appear to be really safe. Based on my experience with these systems, I am confident in their safety and feel assured enough to lessen my concerns about risks, such as oesophageal fistula.
Q: In terms of efficacy, could you describe what has been observed to date?
The efficacy standpoint is a grey zone. The first monocentric or bicentric reports that I have been involved with have been extremely favourable, and we have been studying what we call pulmonary vein reconnection, which occurs when the initial lesion is not durable, and somehow the electrical connection manages to come back between the left atrium and the muscles in the vein, potentially leading to AF recurrences.
To study this, we systematically remapped patients after electroporation for pulmonary vein isolation 3 months after their ablation procedure. The results have been exceptionally positive, with the latest waveform achieving a 96% isolation rate of the veins after 3 months. This represents an unprecedented durability for pulmonary vein isolation. Furthermore, this translated into an 85% success rate at 1 year. However, the latest multicentre report from the ADVENT trial (The FARAPULSE ADVENT PIVOTAL Trial PFA System vs SOC Ablation for Paroxysmal Atrial Fibrillation; ClinicalTrials.gov Identifier: NCT04612244) shows slightly lower figures than these.8Expand Reference The ADVENT trial was a randomized clinical trial that compared FARAPULSE™ PFA with standard-of-care thermal ablation devices (force-sensing RF ablation or cryoballoon ablation) for the treatment of paroxysmal AF.
There are probably some reasons for that, mainly the fact that both in the ADVENT trial and in the BEAT-AF trial, we are comparing electroporation with RF, with the bias that RF has been used for decades, and in contrast, electroporation is a completely new technique in these centres. Therefore, it is interesting that electroporation was not inferior, considering that clinicians had limited time to become accustomed to and learn how to optimize the use of this new technology. In the ongoing BEAT-AF trial, this bias may be less as most centres involved have had the chance to perform at least a few cases using electroporation before enrolling patients.
Q: Can you tell us about the design of the Ground-BrEAking Electroporation-based inTervention for Atrial Fibrillation trial, including the inclusion criteria for participants, the randomization process and the key endpoints being evaluated?
In the BEAT-AF trial, we are examining multiple aspects, but our primary goal is to demonstrate superior efficacy in the standard endpoints used for ablation. Specifically, we aim to measure the percentage of patients who remain free from any type of atrial arrhythmia during a 1-year follow-up period after the initial ablation, although, of course, we also have plenty of other goals and sub-studies to explore.
We largely shared the same inclusion criteria as the ADVENT trial, which also used Boston Scientific’s FARAPULSE™ PFA technology. This was a deliberate decision, as we anticipate eventually merging the two databases. This integration will grant us access to a much larger patient sample, potentially leading to more robust results.
In the BEAT-AF trial, we have two studies: one for paroxysmal AF (BEAT PAROX-AF; n=292; Figure 1) and another for persistent AF (Ground-Breaking Electroporation-based Intervention for PERSistent Atrial Fibrillation Treatment [BEAT PERS-AF]; n=78; Clinical Trials.gov identifier: NCT05418725).9–1191011 The persistent study is a pilot study designed to gather numbers to build a larger study later. For now, we focus on the proximal AF trial, which finished enrolling in mid-January 2024. To qualify for inclusion in this trial, participants had to have documented AF, no previous history of ablation and no conditions that would likely reduce their life expectancy to below 1 year.
Figure 1: Design of the BEAT PAROX-AF randomized clinical trial9Expand Reference
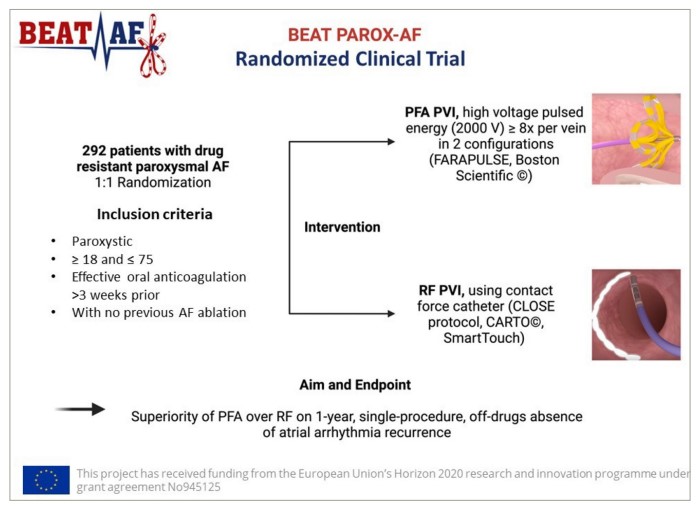
Reproduced from Erhard et al., BEAT-AF Study Group. Comparing pulsed field electroporation and RF ablation for the treatment of paroxysmal AF: design and rationale of the BEAT PAROX-AF randomized clinical trial. Europace. 2024 May 2;26(5):euae103. doi: 10.1093/europace/euae103 under the terms of the Creative Commons CC BY 4.0 International Attribution License (https://creativecommons.org/licenses/by/4.0/).9Expand Reference
AF = atrial fibrillation; BEAT-AF = Ground-BrEAking Electroporation-based inTervention for Atrial Fibrillation; PFA = pulse-field ablation; PVI = pulmonary vein isolation; RF = radiofrequency; V = volts.
These inclusion and exclusion criteria for the trial are considered quite typical. However, what I found fascinating is that despite appearing straightforward and seemingly representative of the population with AF, it actually is not. Using the selected exclusion criteria, it probably resulted in the exclusion of about 80% of the target population, which is a real concern. Ideally, it would be beneficial to conduct a study with only inclusion criteria, so that we could compare with real life and obtain truly representative results.
Regarding the randomization process, in the proximal AF trial, it is 1:1. However, in the persistent trial, because we lack data on electroporation, it is 2:1 in favour of electroporation.
Q: Collaboration among multiple clinical centres across various European countries is a notable aspect of the Ground-BrEAking Electroporation-based inTervention for Atrial Fibrillation trial. Could you briefly describe these collaborations and how you envision they could influence the trial’s outcomes and the subsequent adoption of findings into clinical practice?
There are several aspects here. First, scientifically, it is well recognized that if a study is multicentre, truly multicentre, similar to BEAT-AF, which includes nine centres, you get a completely different picture as compared to when you include one, two or three expert centres. This type of multicentre trial is certainly going to be different.
Typically, the results are not as good, but they are probably way more representative of real life. Another advantage is that it helps establish a network where each centre can significantly influence its own country. Therefore, if the goal is to make a difference across Europe, involving multiple countries in the study is a strategic and effective approach.
Ideally, we should have included more countries and fewer centres per country to broaden the scope of our study. However, given that there is no unified clinical research framework across Europe, this approach poses significant challenges. For example, each country has its own ethics committees and clinical trial-site protocols, which significantly complicate the process of opening new centres and coordinating research efforts. As a result, the BEAT-AF trial is not as ambitious as it could have been. Nonetheless, I believe that it still provides a fairly representative sample of the European population.
Q: How has the Ground-BrEAking Electroporation-based inTervention for Atrial Fibrillation trial progressed since you completed recruitment and what milestones have been achieved so far?
January 2025 will mark the end of the follow-up period for the last patient. Afterwards, we will need a few months to process the data. Once processed, we will freeze the database, meaning it will no longer be subject to changes, and we can begin analysing the data and conducting statistical evaluations. With this timeline in mind, we anticipate having the results by September 2025. At that point, we will start preparing various communication supports, such as writing research articles for publication in journals and creating presentations for key meetings.
Q: Following the 1-year follow-up, are there any further plans?
Conducting long-term follow-up with the participants is a primary focus, given the introduction of this new energy source. Considering the mean age of patients who underwent AF ablation at our centre is between 55 and 60 years, we plan to monitor this cohort for at least 5 years and potentially up to 10 years if feasible. Such extended follow-up is crucial for assessing the long-term safety and effectiveness of the treatment.
Q: Regarding the patients who were excluded from the study, is there a possibility they could be included in future research?
Indeed, we have initiated discussions about how to include those who were previously excluded. Given that over a thousand patients were not eligible under our initial criteria, it is essential to understand and document the reasons for their exclusion. We are considering a dedicated effort to track and report on what happens to these excluded individuals, which could provide valuable insights into the broader applicability and impact of our findings. This approach would not only enhance our understanding of the treatment’s scope but also ensure that we capture a more comprehensive view of the potential benefits and limitations of the new technology.
Q: What is the ultimate goal of the Ground-BrEAking Electroporation-based inTervention for Atrial Fibrillation trial, and how do you see it influencing patient care, clinical guidelines and healthcare efficiency across Europe?
The ultimate goal of the BEAT-AF trial is to advance high-level scientific research in Europe because it truly matters. At the core of all these efforts is the improvement of patient care. Such collaborations are vital for spreading better care practices, particularly in the use of innovative energies for treatment.
Additionally, the impact on clinical guidelines is significant. Medicine is increasingly governed by these guidelines, which are taken extremely seriously by practitioners. If we can demonstrate that catheter ablation using this new energy is a more effective treatment for AF, it could facilitate wider access to this treatment. This is not only due to improved recommendations but also because it represents a more efficient use of healthcare budgets. If the same resources and space can treat twice as many patients effectively, we are not just following guidelines – we are optimizing the financial resources allocated by governments for healthcare. This strategic approach could significantly enhance patient outcomes and the overall efficiency of healthcare systems.
















